Figure 4.
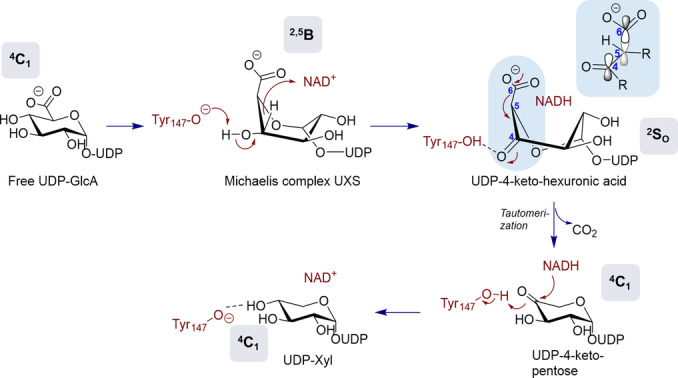
Proposed mechanism of UXS reacting with UDP‐GlcA and yielding UDP‐Xyl. The change in the ring pucker from the initial 4 C 1 chair conformation in UDP‐GlcA to a 2 S O skew‐boat conformation in the UDP‐4‐ketohexuronic acid intermediate brings the carboxylate moiety in an axial orientation, thus resulting in an optimal orbital alignment for rapid decarboxylation.[ 6 , 22 ] See Figure 1 for comparison with UGAepi, which uses stereoelectronic control to prevent the decarboxylation.
