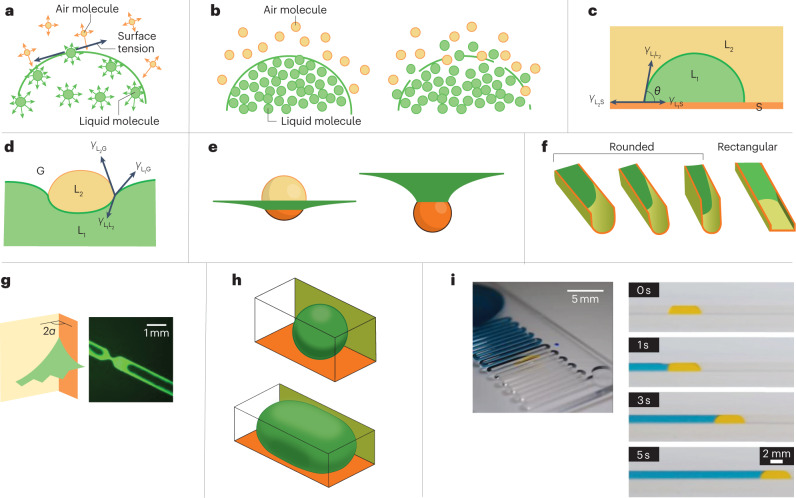
Fig. 2. Forces acting at droplet interfaces.
Key physical considerations and theory that aid the design of open droplet microfluidic systems. a, Asymmetry of molecular interactions at an air–liquid interface results in surface tension. The yellow and green arrows indicate the interactions between air and liquid molecules, respectively. b, Sketches of a sharp (left) or smeared (right) interface, corresponding to high or low surface tension, respectively. c, The Young–Dupré law for a sessile liquid droplet (L1) immersed in an immiscible liquid (L2) on a solid surface (S), where γ is the surface tension and θ is the contact angle. d, The Neumann triangle for the contact of two liquid phases (L1 and L2) and a gas phase (G). e, The dispersed phase (that is, the droplet, shown in yellow and orange) can float on (left) or be immersed in (right) a carrier fluid (green). The different locations of the droplet were calculated using the Surface Evolver software83. f, Different flow behaviours of the carrier fluid (green) in a rounded- or rectangular-bottomed open channel. Capillary filaments can form in rectangular-bottomed channels but not rounded-bottomed ones. The three rounded-bottomed channels show how the aspect (width to height) ratio of the channel affects the meniscus shape of the flow. g, A schematic (left) and photo (right) of the formation of capillary filaments by the carrier fluid in the corners of a rectangular channel, where 2α is the corner interior angle. h, Example of two-phase flow in a channel in which the dispersed liquid phase (that is, droplets and plugs) does not wet the walls75,83, produced using the Surface Evolver software84. i, Photos of a two-phase open channel flow with an aqueous plug (yellow) dispersed in a carrier fluid, 1-nonanol (blue). Liquids were dyed with food colouring for visualization. Part e is adapted with permission from ref. 114, John Wiley & Sons. Part f (left) is adapted with permission from ref. 9, IOP Publishing. Part f (right) is adapted with permission from ref. 5, Wiley-Scrivener. Part g (left) is adapted with permission from ref. 9, IOP Publishing. Part g (right) is adapted from ref. 115 under a Creative Commons licence CC BY 4.0. Part h is adapted with permission from ref. 114, John Wiley & Sons. Part i (left) is reprinted with permission from ref. 9, IOP Publishing. Part i (right) is reprinted with permission from ref. 26, American Chemical Society.