Figure 1.
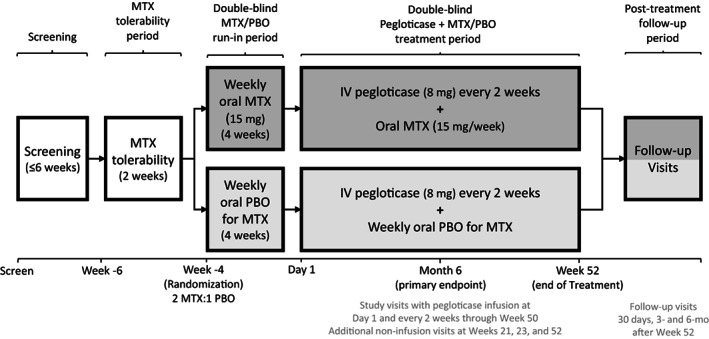
Overview of the study design for assessing pegloticase plus methotrexate (MTX) versus pegloticase plus placebo (PBO) cotreatment for uncontrolled gout in a randomized, placebo‐controlled, double‐blind trial. Following screening, patients underwent a 2‐week MTX tolerability period before randomization then entered a 4‐week run‐in period and 52‐week pegloticase plus MTX or PBO treatment period. Patients with pre‐infusion serum urate >6 mg/dl at 2 consecutive study visits beginning at week 2 discontinued study treatment and remained in the study on observation. Key efficacy and safety assessments were conducted during months 6 and 12.
