Figure 4.
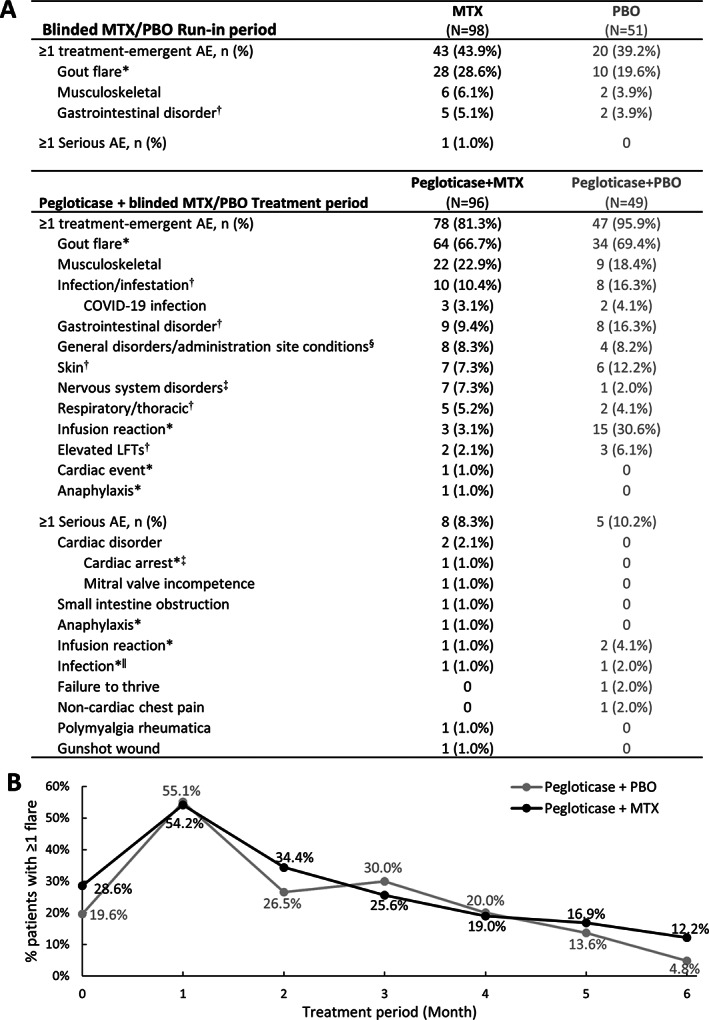
A, Summary of treatment‐emergent adverse events (AEs) occurring in ≥5% of either treatment group through week 24 of the pegloticase plus methotrexate (MTX) or placebo (PBO) treatment period (safety population). All AEs of special interest and serious AEs are also shown. Elevated liver function test (LFT) includes elevated aspartate aminotransferase, alanine aminotransferase, bilirubin, and hepatic enzymes. B, Proportion of patients in each treatment group who experienced ≥1 gout flare during each month of the pegloticase plus MTX or pegloticase plus PBO treatment period. Month 0 represents the proportion of patients with ≥1 flare during the MTX/PBO 4‐week run‐in period. In A, * = AE of special interest, adjudicated. † = known MTX AE (ref. 17). ‡ = nervous system disorders included dizziness (2 MTX), headache (1 MTX, 1 PBO), hypoesthesia (2 MTX), neuropathy peripheral (1 MTX), paresthesia (1 MTX). § = general disorders included fatigue (5 MTX, 1 PBO), chest discomfort (1 PBO), feeling hot (1 MTX), peripheral edema (1 PBO), peripheral swelling (1 MTX), pain (1 MTX), foreign body sensation (1 MTX), non‐cardiac chest pain (1 PBO). ‖ = infections included COVID‐19 (1 MTX) and soft tissue abscess (1 PBO).
