Abstract
Brain plasticity and function is impaired in conditions of metabolic dysregulation, such as obesity. Less is known on whether brain function is also affected by transient and physiological metabolic changes, such as the alternation between fasting and fed state. Here we asked whether these changes affect the transient shift of ocular dominance that follows short‐term monocular deprivation, a form of homeostatic plasticity. We further asked whether variations in three of the main metabolic and hormonal pathways affected in obesity (glucose metabolism, leptin signalling and fatty acid metabolism) correlate with plasticity changes. We measured the effects of 2 h monocular deprivation in three conditions: post‐absorptive state (fasting), after ingestion of a standardised meal and during infusion of glucagon‐like peptide‐1 (GLP‐1), an incretin physiologically released upon meal ingestion that plays a key role in glucose metabolism.
We found that short‐term plasticity was less manifest in fasting than in fed state, whereas GLP‐1 infusion did not elicit reliable changes compared to fasting. Although we confirmed a positive association between plasticity and supraphysiological GLP‐1 levels, achieved by GLP‐1 infusion, we found that none of the parameters linked to glucose metabolism could predict the plasticity reduction in the fasting versus fed state. Instead, this was selectively associated with the increase in plasma beta‐hydroxybutyrate (B‐OH) levels during fasting, which suggests a link between neural function and energy substrates alternative to glucose. These results reveal a previously unexplored link between homeostatic brain plasticity and the physiological changes associated with the daily fast‐fed cycle.
Keywords: binocular rivalry, glucose metabolism, ketone metabolism, ocular‐dominance plasticity, psychophysics
Skipping breakfast impairs short‐term ocular dominance plasticity in adult healthy humans. Replacing a standardised meal with a GLP‐1 infusion does not rescue this plasticity impairment. The plasticity impairment during fasting parallels the enhancement of fatty acids metabolism.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- BMI
body mass index
- B‐OH
beta‐hydroxybutyrate
- DBP
diastolic blood pressure
- Dep
Dominant/Deprived
- GABA
gamma ammino‐butyric acid
- GGT
gamma‐glutamyl transferase
- GLP‐1
glucagon‐like peptide‐1
- Hb
haemoglobin
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- Ndep
non‐dominant/non‐deprived
- RYGB
Roux‐en‐Y gastric bypass
- SBP
systolic blood pressure
1. INTRODUCTION
Metabolic abnormalities and obesity are associated with impaired cognitive performance and learning ability (Tanaka et al., 2020). We recently showed that, in obese individuals, this impairment extends to an aspect of sensory processing: short‐term ocular dominance plasticity (Daniele et al., 2021; Lunghi, Daniele, et al., 2019). This is measured in adult humans, by transiently applying a translucent patch over one eye, that is, monocular deprivation. After deprivation, ocular dominance is transiently but robustly shifted in favour of the deprived eye; this effect can be detected and quantified by comparing ocular dominance before and after deprivation with various psychophysical techniques, including binocular rivalry (Lunghi et al., 2011; Lunghi, Berchicci, et al., 2015; Lunghi, Emir, et al., 2015; Lunghi & Sale, 2015; Lunghi, Sframeli, et al., 2019; Wang et al., 2020) and binocular summation (Min et al., 2018; Zhou et al., 2013, 2014, 2017), and it is interpreted as a form of homeostatic plasticity (Baroncelli & Lunghi, 2021). We and others have shown that the extent of ocular dominance shift varies across individuals (Lunghi, Emir, et al., 2015; Nguyen et al., 2021; Steinwurzel et al., 2020) and correlates with visual properties, such as the strength of inhibitory processing (Steinwurzel et al., 2020). Plasticity also correlates with non‐visual factors, including body mass index (BMI, the ratio between body weight in kilogram and the square of height in metres squared). In individuals with higher BMI, we found reduced ocular dominance plasticity in response to short‐term monocular deprivation, compared to individuals with lower BMI (Lunghi, Daniele, et al., 2019). In morbidly obese individuals (BMI > 30 Kg/m2), this form of ocular dominance plasticity was nearly absent, but it was restored to normal levels following weight loss through Roux‐en‐Y gastric bypass (RYGB) surgery (Daniele et al., 2021).
Because of the complexity of the obesity phenotype, it is difficult to pinpoint the factors underlying these variations of ocular dominance plasticity. Obesity is associated with abnormal signalling in a variety of pathways, inflammation (Guillemot‐Legris & Muccioli, 2017) and gut–brain interactions (Ley et al., 2005). Also, multiple and complex neuronal, hormonal, metabolic adaptations are induced by the marked weight reduction obtained with bariatric surgery. Initial evidence suggested that changes in short‐term ocular dominance plasticity could be associated with changes in a fundamental metabolic factor: glucagon‐like peptide‐1 (GLP‐1). GLP‐1 is secreted by the intestinal L‐cells (Eissele et al., 1992); its levels increase rapidly in response to ingestion of mixed meals, promoting insulin secretion and thereby regulating glucose metabolism. GLP‐1 also acts on several brain areas where its receptors are expressed (Alvarez et al., 2005; Farr et al., 2016; Ten Kulve et al., 2016), and it has been claimed to stimulate neurogenesis as well as to repair and/or reduce neurodegeneration (Glotfelty et al., 2020; Grieco et al., 2019; Holscher, 2022; Katsurada & Yada, 2016). With neuroimaging studies in humans, we found that exenatide, a GLP‐1 receptor agonist, can alter glucose metabolism across the cortex (Daniele et al., 2015) as well as BOLD signals (Binda et al., 2019) in the visual cortex, where it interferes with stimulus preferences (food vs. landscapes), suggesting it might alter visual function. In line with this possibility, we found that the recovery of ocular dominance plasticity following RYGB surgery in morbidly obese patients was associated with the increase of GLP‐1 blood concentrations (Lunghi, Daniele, et al., 2019). This led us to hypothesise that GLP‐1 (dysregulated in morbidly obese patients and strongly enhanced following RYGB surgery) plays an important role in restoring short‐term ocular dominance plasticity in these patients.
It is important to consider that obesity and bariatric surgery interact with a wide range of metabolic pathways, besides those connected with glucose. For instance, there is ample evidence that leptin—the satiety hormone—is dysregulated in morbidly obese patients and that it strongly impacts the brain plasticity potential (Considine et al., 1996; Cui et al., 2017; Enriori et al., 2006; Izquierdo et al., 2019; Maffei & Giordano, 2021; Mainardi et al., 2013; Zhang et al., 1994). There is also evidence that fatty acids metabolism is affected in these patients (Vice et al., 2005). After overnight fasting, liver glycogen stores are depleted and metabolism switches from glycogenolysis to lipolysis; the released free fatty acids are transported into hepatocytes, where they are metabolised with the formation of mainly beta‐hydroxybutyrate (B‐OH) and acetoacetate (Anton et al., 2018). Thus, B‐OH plasma concentrations represent a meaningful index of fatty acid metabolism. Moreover, B‐OH is the preferred source of energy in neuronal cells during fasting (i.e. when glucose availability is reduced), and there is initial evidence that this metabolic switch may be accompanied by altered neuronal function (Longo & Mattson, 2014; Mattson, 2012; Puchalska & Crawford, 2017).
In the present work, we proposed to explore the relationship between neuroplasticity and these diverse metabolic factors by moving away from the pathological model of obesity and focussing on healthy individuals who underwent acute metabolic manipulations. We considered one simple and physiological manipulation: overnight fasting versus ingestion of a (standardised) meal. We compared this with a direct manipulation of GLP‐1 levels, intravenously infused in fasting individuals. Across these three conditions, we measured the strength of ocular dominance plasticity, induced by short‐term monocular deprivation, and the plasma concentration of six parameters that indexed the main metabolic factors of interest: glucose metabolism (indexed by active GLP‐1, insulin and C‐peptide and glucose levels), leptin signalling (measured by circulating leptin levels) and fatty acid metabolism (indexed by B‐OH plasma concentrations).
2. MATERIALS AND METHODS
2.1. Ethics statement
Experimental procedures were approved by the local Ethics committee (Comitato Etico Regionale per la Sperimentazione Clinica, sezione autonoma dell'Area Vasta Nord‐Ovest, Project approval n. 1031 of 15 April 2016) and in line with the Declaration of Helsinki. Participants gave written informed consent before starting the study.
2.2. Participants
Volunteers were invited to access our research facilities for acquiring written informed consent and for a screening visit. A pilot study (described in a dedicated section below) was conducted on a separate cohort to determine the minimum sample size required to reliably detect a reduction of short‐term monocular deprivation effects in the fasting vs. fed state. The results indicated that a sample of N = 11 was sufficient to achieve the pre‐set statistical power of 80%. Based on this analysis, a total of 13 participants were enrolled in the study; 11 participants (6 females) completed all conditions whereas two were unable to complete the study and were discarded from all analyses.
The participants were young and in good health, with no chronic or acute illnesses; they had stable BMI within the physiological range (18.5 kg/m2 < BMI < 30 kg/m2) over the 6 months prior to entering the study. None of the participants took any medications nor engaged in regular vigorous exercise (in the gym or outdoors, as assessed by unstructured self‐report). Table 1 shows a summary of the main anthropometric and clinical features of the study cohort. We relied on the participants' self‐reports for normal vision or adequate and recently updated corrections (contact lenses that they wore during the experiments). Following enrolment, the participants received extensive training on the binocular rivalry task. We verified that they all had typical binocular rivalry dynamics, with low percentage (< = 30%) of mixed percepts (i.e. periods of fusion or superimposition of the two rivalrous stimuli) and no strong ocular dominance (< = 30% dominance difference between eyes).
TABLE 1.
Anthropometric and clinical features of the study cohort (N = 11)
| Sample characteristics | Median (range) |
|---|---|
| Age (years) | 26 (23–38) |
| Gender (F,M) | 6,5 |
| BMI (kg/m2) | 21.48 (18.69–28.41) |
| SBP (mm Hg) | 122 (110–138) |
| DBP (mm Hg) | 80 (55–88) |
| Hb glycated (mmol/mol) | 33 (27–37) |
| Total cholesterol (mg/dl) | 174 (131–211) |
| HDL cholesterol (mg/dl) | 64 (38–79) |
| LDL cholesterol (mg/dl) | 98.2 (53–131) |
| Triglycerides (mg/dl) | 64 (43–202) |
| Creatinine (mg/dl) | 0.75 (0.68–1.24) |
| AST (UI/l) | 19 (13–30) |
| ALT (UI/l) | 13 (7–50) |
| GGT (UI/l) | 14 (8–29) |
| Hb% (g/dl) | 13.6 (10.2–16.5) |
Note: BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, Hb = haemoglobin, HDL = high density lipoprotein, LDL = low density lipoprotein, AST = aspartate aminotransferase, ALT = alanine aminotransferase, GGT = gamma‐glutamyl transferase.
2.3. Experimental procedure
The participants reported to the research centre on three days over the course of about 90 days (minimum interval between visits: 7 days), always at 8 AM after 12 h overnight fasting. A peripheral vein was cannulated for collection of blood samples. Following collection of baseline samples, baseline binocular rivalry measurements were performed (described below). These were followed by the application of a monocular patch over the dominant eye for 2 h, to achieve short‐term monocular deprivation. The monocular deprivation procedure was repeated in three conditions (randomised order):
with the fasting regimen maintained throughout the 2‐h monocular deprivation;
following the administration of a standardised liquid meal (Nutridrink Nutricia, 300 Kcal) immediately followed by the application of the monocular patch;
during a 2‐h intravenous infusion of GLP‐1 (.9 pmol/kg/min) intended to reach (in a fasting regimen) the GLP‐1 concentration usually achieved upon ingestion of a mixed meal. Infusion rate was based on Little et al. (2006). The infusion was initiated at the time of monocular patch application, and it was continued for the 2‐h deprivation period. During the infusion, blood glucose concentration was measured at 10‐min intervals and a 20% D‐glucose (Braun, Melsungen AG, Germany) was intravenously infused at variable rate to maintain fasting plasma glucose levels.
Blood samples were drawn at scheduled intervals 0, 15, 30, 60, 90 and 120 min from the application of the monocular eye patch. The same cannulation procedure was used in all three conditions. It was performed at the beginning of each session and removed after the last post‐deprivation binocular rivalry measurement, by a dedicated expert nurse. Peripheral vein cannulation is the most common method for obtaining vascular access; it entails no needle left in the participants' arm, as only a catheter (a small flexible tube made of synthetic polymers) remains in position during the procedures and it is expected to produce no pain or discomfort as the arm remains free to move. Based on this, we submit that the risk of cannulation affecting the plasticity results is negligible.
2.4. Laboratory assessments
We analysed blood samples to quantify the concentrations of the main hormones and substrates that are expected to undergo physiological changes in the fast‐fed cycle.
To track glucose metabolism, we quantified the concentrations of GLP‐1 (active fraction, measured by Enzyme‐Linked Immunosorbent Assay (ELISA), Merck, Germany), its downstream targets insulin and C‐peptide (measured by RIA, Pantec, Turin, Italy), and the consequent plasma glucose levels (glucose oxidase method, Analox GM9 Analyser; Analox Instruments, London, UK). Insulin and C‐peptide are secreted in equimolar concentrations; insulin regulates glucose metabolism, whereas C‐peptide merely reflects insulin secretion because of its negligible hepatic extraction and constant metabolic clearance rate (Polonsky & Rubenstein, 1984).
We tracked concentrations of leptin using a sandwich ELISA (RayBiotech, Norcross, GA, USA).
Finally, we studied fatty acid metabolism, by tracking concentrations of the main alternative substrate to glucose: beta‐hydroxybutyrate (B‐OH). This was measured with an in‐house automated spectrophotometric enzymatic method on a Beckman UniCel DXC600 Synchron Analyser (Fullerton, CA, USA).
Intra‐assay and between‐assay coefficients of variation were <1% and <5%, respectively, for all hormone and substrate measurements. Data points at 15 and 30 min in the fasting condition were skipped as no notable variations in any of the parameters was expected; for the same reason, GLP‐1 was not quantified at the 15‐min data point. For all parameters, we summarised variations over the 2‐h interval by computing the area under the curve (AUC) using the trapezoidal rule.
2.5. Monocular deprivation procedure
Monocular deprivation was achieved by patching the dominant eye for two hours to induce a shift in ocular dominance as previously described (Lunghi et al., 2011). Recent evidence suggests that shorter deprivation durations may be sufficient to induce a plasticity effect (Min et al., 2018). However, we chose to maintain the 2‐h deprivation schedule because it has been used in previous studies on obesity and bariatric surgery (Daniele et al., 2021; Lunghi, Daniele, et al., 2019), allowing direct comparison. As in those studies, the eye patch was made of a translucent plastic material that allowed light to reach the retina (attenuation 15%) but completely prevented pattern vision (Figure 1a). During the 2‐h deprivation, the participants were asked to remain seated, and they could read or work on their laptops.
FIGURE 1.
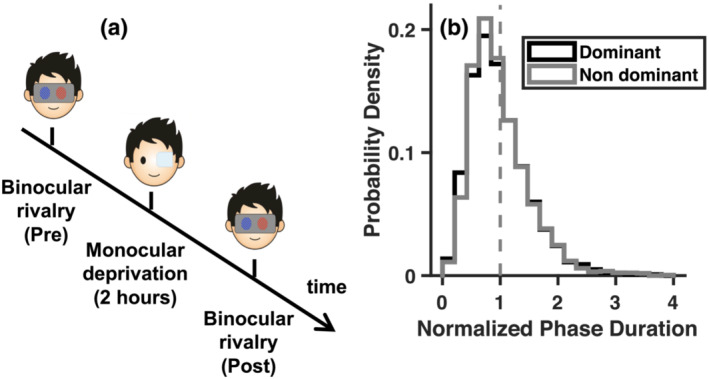
Experimental procedure and baseline binocular rivalry measurements. (a) Time course of the experiment, where binocular rivalry dynamics were measured before and after deprivation. Short‐term monocular deprivation was achieved by applying a translucent patch over the dominant eye for 2 h. (b) Probability density function of the normalised phase durations for each eye (dominant and non‐dominant), measured before deprivation and pooled across conditions and participants. The function conforms to the typical gamma distribution
2.6. Binocular rivalry assessment
Visual stimuli for binocular rivalry were two oblique gratings (orientation: ± 45°, size: 3°, spatial frequency: 2 cpd, contrast 50%), one red and one blue, surrounded by a white smoothed circle and presented against a black uniform background. The peak luminance of the red grating was reduced to match the peak luminance of the blue one using photometric measures. The participants sat in front of the monitor at 57 cm distance (IPS LED 24EA53, with resolution 1920 × 1080, and refresh rate 60 Hz). Because the stimuli were seen through anaglyph red–blue goggles (right lens blue, left lens red), each eye only viewed one of the gratings (with colour matching the lens colour).
Upon stimuli appearance, visual perception started alternating between the two monocular images (clockwise or counter‐clockwise tilted grating), sometimes interleaved with ‘mixed percepts’ in which the two images were either fused or combined in a patch‐wise fashion. The participants were asked to continuously report which of these three states best described their current perception by continuously pressing one of three allocated keyboard keys (right, left, and down arrows) with their right hand. Rivalry was measured over 3‐min long experimental blocks. We acquired two blocks before deprivation, and four blocks immediately after eye‐patch removal. At each experimental block, the orientation of the stimulus in each eye was randomly varied so that the participants did not know which percept (clockwise or counter‐clockwise) was associated with which eye or colour. The recorded perceptual reports were analysed with custom MATLAB scripts. We checked that in all cases phase durations followed the typical gamma distribution. We normalised phase duration for the deprived eye and non‐deprived eye to their mean in each participant; normalised durations were then pooled across the participants and the resulting probability density functions were similar curves for the two eyes, as shown in Figure 1b. We did not include phase durations longer that 30 s (stopped rivalry) or shorter than .3 s (finger errors).
To characterise rivalry dynamics, we used mean phase durations (the mean duration of exclusive dominance phases of the dominant or the non‐dominant eye) and the switch rates, defined as the fraction of perceptual phases (reporting exclusive dominance of dominant or non‐dominant eye, or mixed percepts) observed over a period of 1 s.
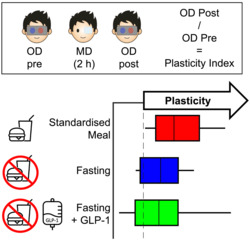
The main purpose of our rivalry measurements was to quantify ocular dominance and monitor its shift following monocular deprivation. We defined ocular dominance as the ratio between mean phase durations for the dominant (Dep) and non‐dominant (Ndep) eye; because we patched the dominant eye, these correspond to the deprived and non‐deprived eye. We then indexed the plasticity effect as the ratio of the ocular dominance index before (Pre) and after (Post) deprivation:
| (1) |
A plasticity index > 1 implies a shift of ocular dominance in favour of the deprived eye (the expected effect). Note that Equation (1) can be rewritten using log‐transformed mean phase durations, becoming a linear combination.
| (2) |
2.7. Statistical analyses
Statistical analyses were performed using MATLAB (MATLAB_R2019a). We checked the distributions of all variables submitted to statistical tests and found that they often significantly deviated from the normality assumption (p < .05 at the Lilliefors test). We applied a logarithmic transform to the AUCs of parameters extracted from blood samples, the binocular rivalry mean phase durations and derived ocular dominance indices, and a z‐transform to the proportions of binocular rivalry mixed percepts (bounded within −3 and +3). With these transformations, all distributions met the normality assumption (again checked with the Lilliefors test).
We used repeated measures analyses of variance (ANOVAs) to evaluate the effects of condition (three levels: fasting, standardised meal and GLP‐1 infusion) and, where appropriate, the effects of factors time (two levels: pre‐ and post‐deprivation) and eye (deprived and non‐deprived). Whenever the sphericity assumption was not met (p < .05 at the Mauchly's test), p values are reported both before and after Greenhouse–Geisser correction. We followed up ANOVAs with a series of post‐hoc paired t tests (single series or contrasts). In particular, we assessed the effect of monocular deprivation on binocular rivalry mean phase durations by means of the contrast defined in Equation (2), which (given the log‐transformation of phase durations) is mathematically equivalent to our plasticity index (Equation 1). The p values resulting from post‐hoc t tests were evaluated against Bonferroni‐corrected alpha levels, obtained by dividing the a priori .05 alpha level by the number of meaningful pairwise comparisons or contrasts (specified for each test). We used the same procedure to evaluate the significance of Pearson's correlations.
For t tests and correlations, we complemented the standard inferential approach with Bayes factors, which quantify the evidence for or against the null hypothesis as the ratio of their likelihoods given the observed data. We expressed Bayes factors as the base‐10 logarithm of this ratio (log‐Bayes Factors or lgBF), where negative numbers indicate that the experimental hypothesis is likely to be false. Conventionally, absolute lgBF greater than .5 are considered ‘substantial’ evidence for or against the experimental hypothesis; absolute lgBF greater than 1 are considered ‘strong’ evidence (Kass & Raftery, 1995).
2.8. Pilot study
We determined the appropriate sample size by means of a pilot experiment, with a different group of participants (N = 10, all females, aged 25.21 [23.50–28] years). We only compared the fasting condition and a rough approximation to the ‘fed’ condition, where instead of providing a standardised meal, we simply asked the participants to have their normal breakfast before reporting to the laboratory for the experiment. Testing always started at the same time (9 AM), and the two conditions were administered in random order, between 7 and 15 days apart. Procedures and analyses were the same as in the main experiment, except that no blood samples were taken and except for the following details. Binocular rivalry was measured with monochromatic stimuli (Gaussian‐vignetted sinusoidal gratings, oriented 45° clockwise or counter‐clockwise, with size: σ = 2°, spatial frequency: 2 cycles/degree of visual angle, and contrast: 50%) seen through ferromagnetic shutter goggles. Stimuli were generated by the ViSaGe (CRS, Cambridge Research Systems), housed in a PC (Dell) controlled by MATLAB scripts. They were displayed on a linearised monitor (Barco CDCT 6551, Barco Federal System, LLC, Duluth, GA) driven at a resolution of 800 × 600 pixels, 140 Hz. Like in the main experiment, the participants were asked to remain seated during the deprivation, reading or working at their laptops. The results showed a significant difference in (log‐transformed) plasticity indices between conditions (Meal‐Fast: t 9 = 2.58, p = .030, lgBF = .42); the effect size was .815 (Cohen's d). We used the software package G*power (G*Power version 3.1.9.6) to calculate the minimum sample size required to measure an effect of this size with 80% power and one‐tailed .05 alpha, which yielded N = 11.
3. RESULTS
3.1. Plasma substrate levels and hormone levels
Figure 2 shows time courses for active GLP‐1, C‐peptide, insulin, glucose, leptin and B‐OH under the three experimental conditions. We used the AUC (log‐transformed) to summarise each time course and entered these values in an ANOVA for repeated measures comparing the three metabolic states (one factor with three levels: fasting, standardised meal and GLP‐1 infusion), followed by post‐hoc tests evaluated against the Bonferroni‐corrected alpha level of .017 (.05 divided by the three possible pairwise comparisons).
FIGURE 2.
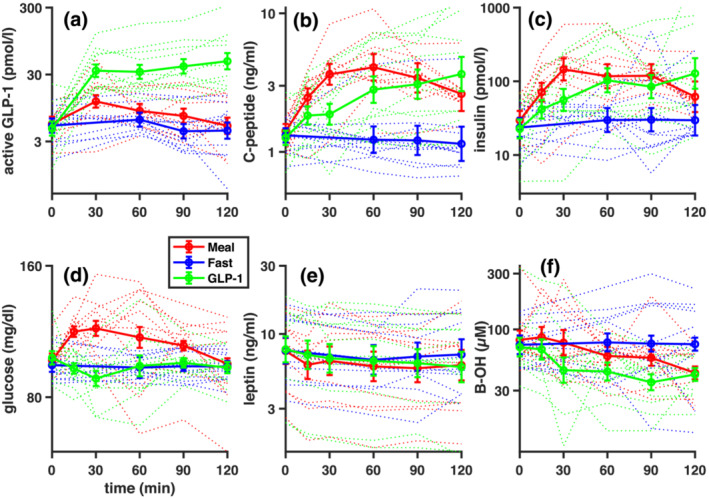
Time courses of metabolic parameters. Plasma concentrations of (a) GLP‐1 (active fraction), (b) C‐peptide, (c) insulin, (d) glucose, (e) leptin and (f) B‐OH over the 2 h of monocular deprivation, separately for the three metabolic states (standardised meal, fasting, fasting plus GLP‐1 infusion). Thick lines show the average, and error bars are the SEM (standard error of the mean across subjects). Thin dotted lines show time courses of metabolites for each participant individually. In all plots, time = 0 min corresponds with the start of the deprivation and the administration of the standardised meal or GLP‐1 infusion
All parameters connected with glucose metabolism showed a significant effect of metabolic state as evaluated with the one‐way ANOVA (GLP‐1: F 2,10 = 24.66, p < .001; C‐peptide: F 2,10 = 10.66, p = .001; insulin: F 2,10 = 4.81, p = .020; glucose: F 2,10 = 13.34, p < .001). Figure 2a shows that ingestion of the standardised meal was followed by an increase of active GLP‐1 levels compared with the fasting condition. At the 30 min timepoint, active GLP‐1 plasma levels upon GLP‐1 infusion were similar to the levels attained in response to the mixed meal ingestion, as expected (Little et al., 2006). However, although the GLP‐1 concentration continued to increase slightly with infusion, it started dropping after reaching the 30‐min peak with the mixed meal. As a result, active GLP‐1 concentrations were significantly different during GLP‐1 infusion than both in the standardised meal condition (t 10 = 4.19, p = .002, lgBF = 1.38) and the fasting conditions (t 10 = 7.03, p < .001, lgBF = 2.84), whereas the smaller and more transient difference between the standardised meal and fasting condition did not reach significance (t 10 = 2.09, p = .063, lgBF = .16).
Figure 2b,c show C‐peptide and insulin levels, which were significantly higher in the standardised meal condition than in the fasting condition (C‐peptide: t 10 = 4.89, p = .001, lgBF = 1.77; insulin: t 10 = 3.66, p = .004, lgBF = 1.07). C‐peptide and insulin levels were more variable in the GLP‐1 infusion condition and non‐significantly different from the other two (all|t 10| < 2.87, all p > .017).
Figure 2d shows that plasma glucose remained at a baseline level in both the fasting condition and during the GLP‐1 infusion, where it was kept constant through titrated glucose infusion, resulting in indistinguishable values in these two conditions (t 10 = .08, p = .934, lgBF = −.53). In contrast, the standardised meal was followed by a transient increase in plasma glucose, resulting in a reliable difference between the standardised meal and the other two conditions (fasting: t 10 = 3.59, p = .005, lgBF = 1.03; GLP‐1 infusion: t 10 = 5.72, p < .001, lgBF = 2.22).
Leptin plasma levels (Figure 2e) were stable and similar in all three experimental conditions, as reported by the non‐significant effect of condition at the one‐way ANOVA (F 2,10 = .59, p = .565) and the non‐significant post‐hoc tests (all |t 10| < 1.23, all p > .250, all lgBF < −.26).
B‐OH levels (Figure 2f) were slightly increased in the fasting condition and decreased after the standardised meal and the GLP‐1 infusion, reported by a significant overall effect of condition at the one‐way ANOVA (F 2,10 = 3.51, p = .049). However, individual pairwise differences did not pass the Bonferroni‐corrected significance threshold (all |t 10| < 2.29, all p > .045, all lgBF < .26).
3.2. Binocular rivalry
We checked the reliability of our binocular rivalry data by comparing pre‐deprivation values collected in the three sessions (in all cases, the participants performed the pre‐deprivation measurements following a 12‐h overnight fasting). We found that all the standard parameters used to describe the rivalry phenomenon were well correlated across conditions (Table 2).
TABLE 2.
Reliability analyses on binocular rivalry measurements (pre‐deprivation)
| Parameter | Conditions | r11 | p value | lgBF |
|---|---|---|---|---|
| Mean phase durations | Meal vs. fast | .92 | <.001 | 2.70 |
| Meal vs. GLP‐1 | .91 | <.001 | 2.48 | |
| Fast vs. GLP‐1 | .80 | .003 | 1.25 | |
| Switch rate | Meal vs. fast | .91 | <.001 | 2.52 |
| Meal vs. GLP‐1 | .88 | <.001 | 2.03 | |
| Fast vs. GLP‐1 | .72 | .013 | .69 | |
| Ocular dominance | Meal vs. fast | .79 | .004 | 1.16 |
| Meal vs. GLP‐1 | .86 | .001 | 1.77 | |
| Fast vs. GLP‐1 | .70 | .016 | .60 |
Mean durations of exclusive dominance phases averaged across eyes (log‐transformed), switch rates (fraction of perceptual phases per second) and ocular dominance indices (ratio of mean phase durations for the dominant and non‐dominant eye, log‐transformed) were measured before deprivation and correlated across the three conditions. The resulting Pearson's r values are reported, together with associated p values and log Bayes factors. Significance is evaluated against the Bonferroni corrected alpha‐level of .017 (.05 divided by the 3 possible pairwise comparisons). All correlations are significant.
We also checked the quality of the rivalry phenomenon in our set of participants, as indexed by the dominance of mixed percepts. We found that mixed percepts dominated for a moderate proportion of total viewing time (<30%). By entering the proportions of mixed percepts (after z‐transform) in a two‐way ANOVA, we found no main effect of time (pre‐ and post‐deprivation: F 1,10 = 3.10, p = .109) and no effect of condition (main effect: F 2,10 = .88, p = .432 and p = .406 after Greenhouse–Geisser correction; time by condition interaction: F 2,10 = .79, p = .469 and p = .444 after Greenhouse–Geisser correction), indicating that they were a stable characteristic of rivalry dynamics.
Finally, we focussed on the main expected effect of monocular deprivation, the ocular dominance shift that we calculated from the change of mean phase durations for the deprived and non‐deprived eye over time. Figure 3a,b compares mean phase durations before and after deprivation separately for each eye. Symbols show data from individual participants, with different colours referring to the three metabolic states. Log‐transformed values were entered a three‐way ANOVA with factors time (pre‐ and post‐deprivation), eye (deprived and non‐deprived) and condition (standardised meal, fasting and GLP‐1 infusion).
FIGURE 3.
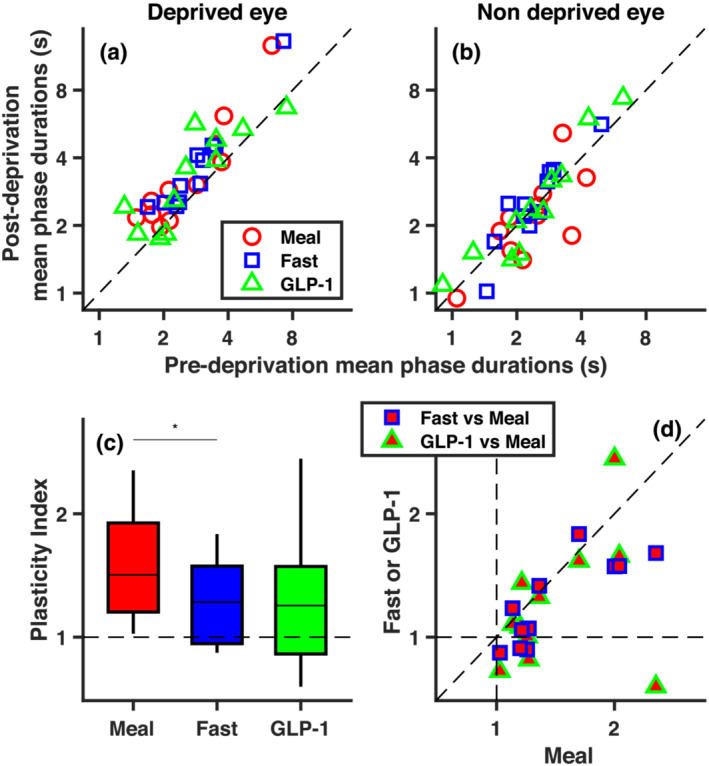
Short‐term ocular dominance plasticity effect. (a, b) Post deprivation vs. pre deprivation mean durations of exclusive dominance phases of the deprived (a) and non‐deprived eye (b), for each participant and for the three metabolic conditions. (c) Box plot of plasticity indices (computed with Equation 1 and summarising ocular dominance shifts following monocular deprivation) in the three conditions. The asterisk above the horizontal lines highlights the only significant paired t test (* for p < .017, corresponding to Bonferroni‐corrected .05 alpha level). (d) Plasticity indices from individual participants in the fasting and GLP‐1 conditions, plotted against plasticity indices in the standardised meal condition. In Figure 3a,b and d, symbols give individual participants results
The significant interaction between factors time and eye supported the statistical reliability of the monocular deprivation effect (time by eye interaction: F 1,10 = 10.28, p = .009; main effect of time: F 1,10 = 21.88, p = .001; main effect of eye: F 1,10 = 6.66, p = .027). Comparison of Figure 3a,b suggests that the deprivation primarily affected mean phase durations for the deprived eye, leaving the non‐deprived eye largely unaffected. Although previous studies generally reported opposite effects of monocular deprivation on the two eyes, we note that the reduction in phase durations for the non‐deprived eye is often (e.g. Binda et al., 2018; Lunghi et al., 2011; Lunghi & Sale, 2015; Ramamurthy & Blaser, 2018; Seeliger & Triesch, 2021; Song et al., 2022; Steinwurzel et al., 2020; Wang et al., 2020; Zhou et al., 2015) though not always (e.g. Lunghi et al., 2013; Lunghi, Berchicci, et al., 2015; Virathone et al., 2021) numerically smaller than the increase for the deprived eye.
To test whether the effect of deprivation varied across conditions (metabolic states), we followed up the non‐significant three‐way interaction (F 2,10 = 2.03, p = .158 and p = .178 after Greenhouse–Geisser correction; and non‐significant condition by time: F 2,10 = .38, p = .690 and p = .688 after Greenhouse–Geisser correction; condition by eye interactions: F 2,10 = .57, p = .572 and p = .543 after Greenhouse–Geisser correction) with planned contrasts comparing the effect of deprivation across the three conditions (evaluated against a Bonferroni‐corrected alpha level of .017 or .05 divided by the three possible pairwise comparisons). Contrasts were defined to be mathematically equivalent to our plasticity index (Equations 1 and 2 in Section 2), shown in Figure 3c for each condition. A significant difference emerged between the standardised meal and fasting conditions (t 10 = 2.90, p = .016, lgBF = .62) with no differences between the GLP‐1 infusion and the other two conditions (all|t 10| < 1.48 and all p > .17). Coherently, the effect of deprivation passed the Bonferroni‐corrected significance threshold (p < .017) only in the standardised meal condition (t 10 = 3.79, p = .004, lgBF = 1.15), not in the fasting condition (t 10 = 2.72, p = .022, lgBF = .52) or in the GLP‐1 infusion condition (t 10 = 1.61, p = .138, lgBF = −.09). Figure 3d directly compares the plasticity index across conditions for the individual participants. The x‐axis reports results from the standardised meal condition; all points lay to the right of the x = 1 line, implying the expected deprivation effect. This is not the case for the y axis, which shows results from the other two conditions and where several points lay below the y = 1 line. Plasticity indices in the fasting and standardised meal condition were well correlated across the participants (r 11 = .82, p = .002, lgBF = 1.41), supporting the validity of this psychophysical measure. Values in the fasting conditions were smaller than in the standardised meal condition in most participants, resulting in most red/blue points laying below the bisection line and only three points abutting this line. Plasticity indices in the GLP‐1 infusion condition had larger variance; one participant showed a large positive effect in the standardised meal and fasting conditions, but an opposite effect in the GLP‐1 infusion condition. This produced non‐significant correlations of the GLP‐1 infusion condition with the other two conditions (GLP‐1 vs. meal: r 11 = .34, p = .314, lgBF = −.43; GLP‐1 vs. fast: r 11 = .46, p = .150, lgBF = −.20).
In our search for potential factors accounting for the acute plasticity change (fasting vs. standardised meal), we looked for possible relationships with plasma levels of hormones and substrates, quantified as their AUCs. Significance of correlations was evaluated against the Bonferroni‐corrected alpha level of .008 (or .05 divided by the six possible correlations between plasticity indices and plasma levels). A significant inverse correlation emerged between the ratio of plasticity indices in the standardised meal vs. fasting conditions and the ratio of AUCs for B‐OH in the same conditions (r 11 = −.84, p = .001, lgBF = 1.58; Figure 4a), whereas there was no association with the ratios of AUCs for any of the other parameters (plasma glucose, insulin, C‐peptide, active GLP‐1 or leptin, all|r| < .35 and p > .288). Reiterating the finding in Figure 3d, Figure 4a shows that there were only three participants with (slightly) weaker plasticity in the standardised meal than in the fasting condition (the three points below the y = 1 line). The same three participants also showed higher B‐OH concentrations in the standardised meal than in the fasting condition.
FIGURE 4.
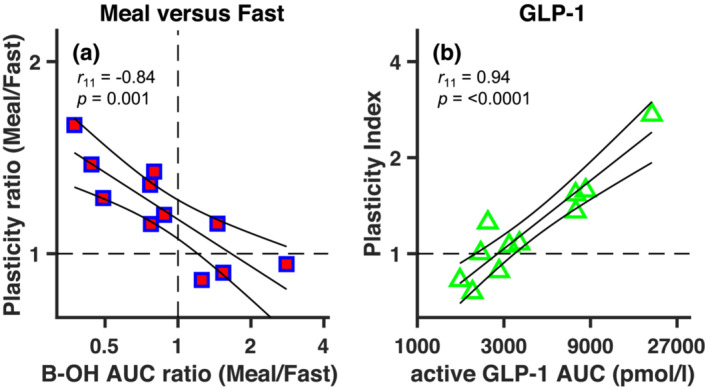
Relationship between metabolic parameters and ocular dominance plasticity. (a) The ratio of plasticity index values in the standardised meal and the fasting condition (y axis) is negatively correlated with the ratio in plasma concentrations of B‐OH (quantified as AUCs) in the two conditions. (b) The plasticity index in the GLP‐1 infusion condition is positively correlated with the plasma concentration of active GLP‐1 (AUCs) in the same condition. In all panels, symbols give individual participants results and continuous lines show the linear regression through the points with associated 95% confidence intervals
We next sought to investigate the origin of the large inter‐individual variability in the GLP‐1 condition. Although GLP‐1 was not associated with plasticity in the fasting or fed state, the much higher, supraphysiological levels of active GLP‐1 achieved through GLP‐1 infusion were strongly and positively correlated with plasticity indexes in this condition (r 11 = .94, p < .0001, lgBF = 3.12; Figure 4b), whereas correlations with the other parameters were non‐significant (all|r| < .50 and p > .122 except for insulin: r 11 = .71, p = .015, lgBF = .63, which did not survive Bonferroni correction). Note that in Figure 4b all data‐points lay close to the best‐fitting regression line; the participant showing very low plasticity in the GLP‐1 infusion condition (the clear outlier in Figure 3d) is not an outlier in this representation because of the low active GLP‐1 concentrations reached upon infusion.
Although we acknowledge the explorative nature of our correlation analyses, given our relatively small sample size, we highlight the strength of both GLP‐1 and B‐OH correlations with our plasticity index, testified by the small p values (implying statistical significance after Bonferroni correction) and supported by our Bayesian analysis, as associated lgBF are > 1, indicative of ‘strong’ evidence in support of the reported associations (Kass & Raftery, 1995).
4. DISCUSSION
By measuring the ocular dominance shift that follows 2‐h monocular deprivation, we found that short‐term ocular dominance plasticity was reduced after overnight fasting compared to after a standardised meal. This modulation of ocular dominance suggests that hormonal and/or metabolic factors can exert an acute modulation of homeostatic plasticity, which is sufficiently rapid to manifest itself over the 2 hours of our protocol—similar to the acute effects of other non‐visual factors, such as physical exercise (Lunghi & Sale, 2015; Lunghi, Sframeli, et al., 2019; but see Finn et al., 2019; Virathone et al., 2021) and pharmacologic manipulations (Sheynin et al., 2019), and in stark contrast with the chronic effects of obesity and bariatric surgery (Daniele et al., 2021; Lunghi, Daniele, et al., 2019).
There is ample evidence for the impact of dietary regimes on brain function and health (Arnold et al., 2014; Longo & Mattson, 2014). However, most studies have focussed on pathological conditions and on long‐term manipulations (e.g. days or months of altered dietary regimens). For instance, there is evidence that obesity has deleterious effects on cognition and neuroplasticity, whereas caloric restriction or bariatric surgery promote neuroplasticity (Mainardi et al., 2013; Murphy et al., 2014), even in primary sensory cortex (Spolidoro et al., 2011). In all these conditions, however, it is difficult to ascertain whether the effects are produced by metabolic shifts, chronic hormonal responses or long‐term consequences on other systems, like, for instance, the immune system (de Heredia et al., 2012; Guillemot‐Legris & Muccioli, 2017) and/or the gut microbiome (Ley et al., 2005).
Fewer studies have tested the acute effects of a metabolic intervention, the majority of which focussed on skipping breakfast. Benau et al. (2014) reviewed findings from 10 studies, most of which reported a negative impact of meal skipping on task performance, for example, short‐term verbal and non‐verbal memory (Benton & Parker, 1998; Sünram‐Lea et al., 2001), long‐term memory (Sünram‐Lea et al., 2001), working memory (Benton & Parker, 1998) and psychomotor speed (Green et al., 1997). Later studies further supported the concept that short‐term fasting negatively affects cognitive performance (Anderson et al., 2018; Komiyama et al., 2016; Pena‐Jorquera et al., 2021). There is also evidence that meal skipping impacts visual perception, reducing the susceptibility to size illusions (Zitron‐Emanuel & Ganel, 2018, 2020) and affecting the idiosyncratic preference for local or global processing (Pender et al., 2014). However, findings from different studies are partially inconsistent, possibly because of the heterogeneity of methods used for assessing performance. Another issue could be the difficulty of controlling for confounding factors, like motivation and arousal, that are likely to impact cognitive function.
The approach we adopted here has the advantage of relying on simple well‐validated sensory measurements, which are less likely to be affected by these confounders. For example, although motivation could affect the precision of perceptual reports during binocular rivalry, it is unlikely to bias ocular dominance estimate, as these are indirectly derived from perceptual reports by combining data across multiple binocular rivalry sessions. Importantly, we could directly quantify the reliability of perceptual reports and of our ocular dominance estimates using a test–retest reliability approach, which showed excellent concordance of our measurements across sessions.
Another advantage of our approach is that it offered insights into the physiological mechanisms that might follow the daily cycle of fasting and fed states. An obvious candidate is glucose metabolism, as indexed by changes in plasma levels or glucose, insulin, and incretin hormones. Both insulin and GLP‐1 have well‐documented effects in the central nervous system (Alvarez et al., 2005; Schulingkamp et al., 2000). The increase in the circulating levels of GLP‐1 has been suggested to contribute to the increase of ocular dominance plasticity following bariatric surgery (Daniele et al., 2021). Nonetheless, in the non‐obese individuals enrolled in the present study, we found no correlation between plasticity effects and any of these key players in glucose homeostasis. Coherently, we found that raising fasting GLP‐1 levels through direct infusion did not modify the plasticity levels, which remained undistinguishable from those recorded under fasting—although variability in the supraphysiological concentrations of active GLP‐1 achieved upon GLP‐1 infusion did associate with inter‐individual variability in short‐term plasticity (Figure 4b), providing a possible explanation for the lack of correlation between plasticity effects in the GLP‐1 infusion condition and the other two conditions (Figure 3d). The GLP‐1 levels reached upon infusion in the present study were in the same order of magnitude as in Daniele et al. (2021). However, in this case, the GLP‐1 increase was only produced acutely during the short‐term monocular deprivation. In contrast, patients in Daniele et al. (2021) were chronically exposed to such high GLP‐1 levels, reached after every meal. Taken together, these results suggest that chronically maintained supraphysiological GLP‐1 levels may be required to significantly modulate brain plasticity, whereas an acute raise of GLP‐1 levels is insufficient to generate a net plasticity change.
Of interest, we found preliminary evidence that neuroplasticity may be modulated by energy substrates alternative to glucose. Our primary outcome, the plasticity reduction in the fasting condition, was correlated with the increase of B‐OH concentrations in the fasting condition (both values compared to the standardised meal condition). This is consistent with recent work in animals indicating a role of B‐OH in multiple brain functions (Achanta & Rae, 2017; Jensen et al., 2020), including plasticity (Jensen et al., 2020; Mattson et al., 2018). Besides affecting metabolic efficiency and ATP availability in neurons, ketone bodies could also directly affect neurotransmission by regulating the storage and release of Gamma Ammino‐Butyric Acid (GABA), the primary inhibitory neurotransmitter in the adult brain (Daikhin & Yudkoff, 1998; Yudkoff et al., 2001). GABA has been directly implicated in neuroplasticity. Neuroplasticity comes in many forms. Hebbian plasticity is usually associated with potentiation or depression of synapses, such as those leading to long‐term changes in ocular dominance following monocular deprivation in the developing brain (Hensch, 2005). Homeostatic plasticity is associated with short‐term changes in response gain (Turrigiano & Nelson, 2004), such as those observed following short‐term monocular deprivation in the adult. Albeit involving completely different cellular mechanisms, both of these forms of plasticity have been linked with GABAergic signalling (Hensch, 2005; Turrigiano & Nelson, 2004). Using the same short‐term ocular dominance plasticity paradigm used here, some of us have previously shown that GABA concentration in occipital cortex decreases after 2 h of monocular deprivation, and that GABA changes correlate with the ocular dominance plasticity effect (Lunghi, Emir, et al., 2015). Based on these observations, we speculate that the moderate increase in B‐OH concentration, occurring during short‐term prolongation of the overnight fasting, may be at least partially responsible for the reduced ocular dominance plasticity, possibly through an enhancement of GABAergic inhibition in the visual cortex.
In conclusion, we report an unprecedented attempt to explore the impact of metabolic and hormonal changes occurring during the daily cycle of fasting and fed states on ocular dominance plasticity in healthy humans. Our results indicate reduced plasticity in the post‐absorptive state that can rapidly switch to enhanced visual plasticity in response to the ingestion of a meal. Further studies will be required to shed light on the mechanisms accounting for this effect; from observations in our limited sample, a role of ketone bodies and alternative energy substrate emerge as an interesting clue.
AUTHOR CONTRIBUTIONS
Silvia Animali: Investigation, Data analyses, Results interpretation, Writing‐original draft. Cecilia Steinwurzel: Investigation, Data analyses, Results interpretation, Writing‐review and editing. Angela Dardano: Investigation. Veronica Sancho‐Bornez: Investigation. Stefano Del Prato: Conceptualisation, Results interpretation, Writing‐review and editing. Maria Concetta Morrone: Conceptualisation, Results interpretation, Writing‐review and editing. Giuseppe Daniele: Conceptualisation, Results interpretation, Writing‐review and editing. Paola Binda: Conceptualisation, Data analyses, Results interpretation, Writing‐original draft, Writing‐review and editing. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15873.
ACKNOWLEDGEMENTS
This work was supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme, grant no. 801715 (PUPILTRAITS) and grant no. 832813 (GenPercept), and by the Italian Ministry of University and Research under the PRIN2017 programme (grant MISMATCH), PNRR‐THE (Tuscany Health Ecosystem, milestone 9.1) and FARE‐2 (grant SMILY). We thank Dr. Claudia Lunghi for contributing to the pilots of this study.
Animali, S. , Steinwurzel, C. , Dardano, A. , Sancho‐Bornez, V. , Del Prato, S. , Morrone, M. C. , Daniele, G. , & Binda, P. (2023). Effect of fasting on short‐term visual plasticity in adult humans. European Journal of Neuroscience, 57(1), 148–162. 10.1111/ejn.15873
Edited by: Martin Giurfa
Funding information H2020 European Research Council, Grant/Award Numbers: 801715 (PUPILTRAITS), 832813 (GenPercept); Ministero dell'Università e della Ricerca, Grant/Award Numbers: FARE‐2 (grant SMILY), PNRR‐THE (Tuscany Health Ecosystem) Milestone 9.1, PRIN2017 (grant MISMATCH); European Research Council (ERC), Grant/Award Numbers: 832813, 801715; Italian Ministry of University and Research, Grant/Award Numbers: SMILY, MISMATCH
Contributor Information
Giuseppe Daniele, Email: giuseppe.daniele@unipi.it.
Paola Binda, Email: paola.binda@unipi.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available at 10.5281/zenodo.7360737.
REFERENCES
- Achanta, L. B. , & Rae, C. D. (2017). Beta‐Hydroxybutyrate in the brain: One molecule, multiple mechanisms. Neurochemical Research, 42, 35–49. 10.1007/s11064-016-2099-2 [DOI] [PubMed] [Google Scholar]
- Alvarez, E. , Martinez, M. D. , Roncero, I. , Chowen, J. A. , Garcia‐Cuartero, B. , Gispert, J. D. , Sanz, C. , Vazquez, P. , Maldonado, A. , de Caceres, J. , Desco, M. , Pozo, M. A. , & Blazquez, E. (2005). The expression of GLP‐1 receptor mRNA and protein allows the effect of GLP‐1 on glucose metabolism in the human hypothalamus and brainstem. Journal of Neurochemistry, 92, 798–806. 10.1111/j.1471-4159.2004.02914.x [DOI] [PubMed] [Google Scholar]
- Anderson, J. R. , Hawkins, M. A. W. , Updegraff, J. , Gunstad, J. , & Spitznagel, M. B. (2018). Baseline glucoregulatory function moderates the effect of dairy milk and fruit juice on postprandial cognition in healthy young adults. European Journal of Nutrition, 57, 2343–2352. 10.1007/s00394-017-1505-0 [DOI] [PubMed] [Google Scholar]
- Anton, S. D. , Moehl, K. , Donahoo, W. T. , Marosi, K. , Lee, S. A. , Mainous, A. G. , Leeuwenburgh, C. , & Mattson, M. P. (2018). Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity, 26, 254–268. 10.1002/oby.22065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, S. E. , Lucki, I. , Brookshire, B. R. , Carlson, G. C. , Browne, C. A. , Kazi, H. , Bang, S. , Choi, B. R. , Chen, Y. , McMullen, M. F. , & Kim, S. F. (2014). High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiology of Disease, 67, 79–87. 10.1016/j.nbd.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli, L. , & Lunghi, C. (2021). Neuroplasticity of the visual cortex: In sickness and in health. Experimental Neurology, 335, 113515. 10.1016/j.expneurol.2020.113515 [DOI] [PubMed] [Google Scholar]
- Benau, E. M. , Orloff, N. C. , Janke, E. A. , Serpell, L. , & Timko, C. A. (2014). A systematic review of the effects of experimental fasting on cognition. Appetite, 77, 52–61. 10.1016/j.appet.2014.02.014 [DOI] [PubMed] [Google Scholar]
- Benton, D. , & Parker, P. Y. (1998). Breakfast, blood glucose, and cognition. The American Journal of Clinical Nutrition, 67, 772s–778s. 10.1093/ajcn/67.4.772S [DOI] [PubMed] [Google Scholar]
- Binda, P. , Eldor, R. , Huerta, C. , Adams, J. , Lancaster, J. , Fox, P. , Del Prato, S. , DeFronzo, R. , Abdul‐Ghani, M. , & Daniele, G. (2019). Exenatide modulates visual cortex responses. Diabetes/Metabolism Reviews, 35, e3167. 10.1002/dmrr.3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda, P. , Kurzawski, J. W. , Lunghi, C. , Biagi, L. , Tosetti, M. , & Morrone, M. C. (2018). Response to short‐term deprivation of the human adult visual cortex measured with 7T BOLD. eLife, 7, e40014. 10.7554/eLife.40014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine, R. V. , Sinha, M. K. , Heiman, M. L. , Kriauciunas, A. , Stephens, T. W. , Nyce, M. R. , Ohannesian, J. P. , Marco, C. C. , McKee, L. J. , Bauer, T. L. , & Caro, J. F. (1996). Serum immunoreactive leptin concentrations in normal‐weight and obese humans. New England Journal of Medicine, 334, 292–295. 10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- Cui, H. X. , Lopez, M. , & Rahmouni, K. (2017). The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nature Reviews. Endocrinology, 13, 338–351. 10.1038/nrendo.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikhin, Y. , & Yudkoff, M. (1998). Ketone bodies and brain glutamate and GABA metabolism. Developmental Neuroscience, 20, 358–364. 10.1159/000017331 [DOI] [PubMed] [Google Scholar]
- Daniele, G. , Iozzo, P. , Molina‐Carrion, M. , Lancaster, J. , Ciociaro, D. , Cersosimo, E. , Tripathy, D. , Triplitt, C. , Fox, P. , Musi, N. , DeFronzo, R. , & Gastaldelli, A. (2015). Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes, 64, 3406–3412. 10.2337/db14-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele, G. , Lunghi, C. , Dardano, A. , Binda, P. , Ceccarini, G. , Santini, F. , Giusti, L. , Ciccarone, A. , Bellini, R. , Moretto, C. , Morrone, M. C. , & Del Prato, S. (2021). Bariatric surgery restores visual cortical plasticity in nondiabetic subjects with obesity. International Journal of Obesity, 45, 1821–1829. 10.1038/s41366-021-00851-0 [DOI] [PubMed] [Google Scholar]
- de Heredia, F. P. , Gomez‐Martinez, S. , & Marcos, A. (2012). Obesity, inflammation and the immune system. The Proceedings of the Nutrition Society, 71, 332–338. 10.1017/S0029665112000092 [DOI] [PubMed] [Google Scholar]
- Eissele, R. , Goke, R. , Willemer, S. , Harthus, H. P. , Vermeer, H. , Arnold, R. , & Goke, B. (1992). Glucagon‐like peptide‐1 cells in the gastrointestinal‐tract and pancreas of rat, pig and man. European Journal of Clinical Investigation, 22, 283–291. 10.1111/j.1365-2362.1992.tb01464.x [DOI] [PubMed] [Google Scholar]
- Enriori, P. J. , Evans, A. E. , Sinnayah, P. , & Cowley, M. A. (2006). Leptin resistance and obesity. Obesity, 14, 254S–258S. 10.1038/oby.2006.319 [DOI] [PubMed] [Google Scholar]
- Farr, O. M. , Sofopoulos, M. , Tsoukas, M. A. , Dincer, F. , Thakkar, B. , Sahin‐Efe, A. , Filippaios, A. , Bowers, J. , Srnka, A. , Gavrieli, A. , Ko, B. J. , Liakou, C. , Kanyuch, N. , Tseleni‐Balafouta, S. , & Mantzoros, C. S. (2016). GLP‐1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP‐1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo‐controlled trial. Diabetologia, 59, 954–965. 10.1007/s00125-016-3874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, A. E. , Baldwin, A. S. , Reynaud, A. , & Hess, R. F. (2019). Visual plasticity and exercise revisited: No evidence for a “cycling lane”. Journal of Vision, 19, 21. 10.1167/19.6.21 [DOI] [PubMed] [Google Scholar]
- Glotfelty, E. J. , Olson, L. , Karlsson, T. E. , Li, Y. Z. , & Greig, N. H. (2020). Glucagon‐like peptide‐1 (GLP‐1)‐based receptor agonists as a treatment for Parkinson's disease. Expert Opinion on Investigational Drugs, 29, 595–602. 10.1080/13543784.2020.1764534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. W. , Elliman, N. A. , & Rogers, P. J. (1997). The effects of food deprivation and incentive motivation on blood glucose levels and cognitive function. Psychopharmacology, 134, 88–94. 10.1007/s002130050429 [DOI] [PubMed] [Google Scholar]
- Grieco, M. , Giorgi, A. , Gentile, M. C. , d'Erme, M. , Morano, S. , Maras, B. , & Filardi, T. (2019). Glucagon‐like peptide‐1: A focus on neurodegenerative diseases. Frontiers in Neuroscience, 13, 1112. 10.3389/fnins.2019.01112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot‐Legris, O. , & Muccioli, G. G. (2017). Obesity‐induced neuroinflammation: Beyond the hypothalamus. Trends in Neurosciences, 40, 237–253. 10.1016/j.tins.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nature Reviews. Neuroscience, 6, 877–888. 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- Holscher, C. (2022). Protective properties of GLP‐1 and associated peptide hormones in neurodegenerative disorders. British Journal of Pharmacology, 179, 695–714. 10.1111/bph.15508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, A. G. , Crujeiras, A. B. , Casanueva, F. F. , & Carreira, M. C. (2019). Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients, 11, 2704. 10.3390/nu11112704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, N. J. , Wodschow, H. Z. , Nilsson, M. , & Rungby, J. (2020). Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. International Journal of Molecular Sciences, 21, 8767. 10.3390/ijms21228767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, R. E. , & Raftery, A. E. (1995). Bayes factors. Journal of the American Statistical Association, 90, 773–795. 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Katsurada, K. , & Yada, T. (2016). Neural effects of gut‐ and brain‐derived glucagon‐like peptide‐1 and its receptor agonist. Journal of Diabetes Investigation, 7, 64–69. 10.1111/jdi.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama, T. , Sudo, M. , Okuda, N. , Yasuno, T. , Kiyonaga, A. , Tanaka, H. , Higaki, Y. , & Ando, S. (2016). Cognitive function at rest and during exercise following breakfast omission. Physiology & Behavior, 157, 178–184. 10.1016/j.physbeh.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Ley, R. E. , Backhed, F. , Turnbaugh, P. , Lozupone, C. A. , Knight, R. D. , & Gordon, J. I. (2005). Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences, 102, 11070–11075. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, T. J. , Pilichiewicz, A. N. , Russo, A. , Phillips, L. , Jones, K. L. , Nauck, M. A. , Wishart, J. , Horowitz, M. , & Feinle‐Bisset, C. (2006). Effects of intravenous glucagon‐like peptide‐1 on gastric emptying and intragastric distribution in healthy subjects: Relationships with postprandial glycemic and insulinemic responses. The Journal of Clinical Endocrinology and Metabolism, 91, 1916–1923. 10.1210/jc.2005-2220 [DOI] [PubMed] [Google Scholar]
- Longo, V. D. , & Mattson, M. P. (2014). Fasting: Molecular mechanisms and clinical applications. Cell Metabolism, 19, 181–192. 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C. , Berchicci, M. , Morrone, M. C. , & Di Russo, F. (2015). Short‐term monocular deprivation alters early components of visual evoked potentials. The Journal of Physiology, 593, 4361–4372. 10.1113/JP270950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C. , Burr, D. C. , & Morrone, C. (2011). Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Current Biology, 21, R538–R539. 10.1016/j.cub.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Lunghi, C. , Burr, D. C. , & Morrone, M. C. (2013). Long‐term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. Journal of Vision, 13, 1. 10.1167/13.6.1 [DOI] [PubMed] [Google Scholar]
- Lunghi, C. , Daniele, G. , Binda, P. , Dardano, A. , Ceccarini, G. , Santini, F. , Del Prato, S. , & Morrone, M. C. (2019). Altered visual plasticity in morbidly obese subjects. Iscience, 22, 206–213. 10.1016/j.isci.2019.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C. , Emir, U. E. , Morrone, M. C. , & Bridge, H. (2015). Short‐term monocular deprivation alters GABA in the adult human visual cortex. Current Biology, 25, 1496–1501. 10.1016/j.cub.2015.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C. , & Sale, A. (2015). A cycling lane for brain rewiring. Current Biology, 25, R1122–R1123. 10.1016/j.cub.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C. , Sframeli, A. T. , Lepri, A. , Lepri, M. , Lisi, D. , Sale, A. , & Morrone, M. C. (2019). A new counterintuitive training for adult amblyopia. Annals of Clinical Translational Neurology, 6, 274–284. 10.1002/acn3.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei, M. , & Giordano, A. (2021). Leptin, the brain and energy homeostasis: From an apparently simple to a highly complex neuronal system. Reviews in Endocrine & Metabolic Disorders, 23, 87–101. 10.1007/s11154-021-09636-2 [DOI] [PubMed] [Google Scholar]
- Mainardi, M. , Pizzorusso, T. , & Maffei, M. (2013). Environment, leptin sensitivity, and hypothalamic plasticity. Neural Plasticity, 2013, 438072. 10.1155/2013/438072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M. P. (2012). Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metabolism, 16, 706–722. 10.1016/j.cmet.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M. P. , Moehl, K. , Ghena, N. , Schmaedick, M. , & Cheng, A. W. (2018). Intermittent metabolic switching, neuroplasticity and brain health. Nature Reviews. Neuroscience, 19, 81–94. 10.1038/nrn.2017.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, S. H. , Baldwin, A. S. , Reynaud, A. , & Hess, R. F. (2018). The shift in ocular dominance from short‐term monocular deprivation exhibits no dependence on duration of deprivation. Scientific Reports, 8, 17083. 10.1038/s41598-018-35084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T. , Dias, G. P. , & Thuret, S. (2014). Effects of diet on brain plasticity in animal and human studies: Mind the gap. Neural Plasticity, 2014, 563160. 10.1155/2014/563160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, B. N. , Malavita, M. , Carter, O. L. , & McKendrick, A. M. (2021). Neuroplasticity in older adults revealed by temporary occlusion of one eye. Cortex, 143, 1–11. 10.1016/j.cortex.2021.07.004 [DOI] [PubMed] [Google Scholar]
- Pena‐Jorquera, H. , Campos‐Nunez, V. , Sadarangani, K. P. , Ferrari, G. , Jorquera‐Aguilera, C. , & Cristi‐Montero, C. (2021). Breakfast: A crucial meal for adolescents' cognitive performance according to their nutritional status. The cogni‐action project. Nutrients, 13, 1320. 10.3390/nu13041320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender, S. , Gilbert, S. J. , & Serpell, L. (2014). The neuropsychology of starvation: Set‐shifting and central coherence in a fasted nonclinical sample. PLoS ONE, 9, e110743. 10.1371/journal.pone.0110743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky, K. S. , & Rubenstein, A. H. (1984). C‐peptide as a measure of the secretion and hepatic extraction of insulin—Pitfalls and limitations. Diabetes, 33, 486–494. 10.2337/diab.33.5.486 [DOI] [PubMed] [Google Scholar]
- Puchalska, P. , & Crawford, P. A. (2017). Multi‐dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metabolism, 25, 262–284. 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy, M. , & Blaser, E. (2018). Assessing the kaleidoscope of monocular deprivation effects. Journal of Vision, 18, 14. 10.1167/18.13.14 [DOI] [PubMed] [Google Scholar]
- Schulingkamp, R. J. , Pagano, T. C. , Hung, D. , & Raffa, R. B. (2000). Insulin receptors and insulin action in the brain: Review and clinical implications. Neuroscience and Biobehavioral Reviews, 24, 855–872. 10.1016/S0149-7634(00)00040-3 [DOI] [PubMed] [Google Scholar]
- Seeliger, N. , & Triesch, J. (2021) A Computational Model of the Effect of Short‐Term Monocular Deprivation on Binocular Rivalry in the Context of Amblyopia. Lect Notes Comput Sc, 12891, 593–603. 10.1007/978-3-030-86362-3_48 [DOI]
- Sheynin, Y. , Chamoun, M. , Baldwin, A. S. , Rosa‐Neto, P. , Hess, R. F. , & Vaucher, E. (2019). Cholinergic potentiation alters perceptual eye dominance plasticity induced by a few hours of monocular patching in adults. Frontiers in Neuroscience, 13, 22. 10.3389/fnins.2019.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, F. X. , Lyu, L. L. , Zhao, J. X. , & Bao, M. (2022). The role of eye‐specific attention in ocular dominance plasticity. Cerebral Cortex, bhac116. 10.1093/cercor/bhac116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro, M. , Baroncelli, L. , Putignano, E. , Maya‐Vetencourt, J. F. , Viegi, A. , & Maffei, L. (2011). Food restriction enhances visual cortex plasticity in adulthood. Nature Communications, 2(1), 320. 10.1038/ncomms1323 [DOI] [PubMed] [Google Scholar]
- Steinwurzel, C. , Animali, S. , Cicchini, G. M. , Morrone, M. C. , & Binda, P. (2020). Using psychophysical performance to predict short‐term ocular dominance plasticity in human adults. Journal of Vision, 20, 6. 10.1167/jov.20.7.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sünram‐Lea, S. , Foster, J. K. , Durlach, P. , & Perez, C. (2001). Glucose facilitation of cognitive performance in healthy young adults: Examination of the influence of fast‐duration, time of day and pre‐consumption plasma glucose levels. Psychopharmacology, 157, 46–54. 10.1007/s002130100771 [DOI] [PubMed] [Google Scholar]
- Tanaka, H. , Gourley, D. D. , Dekhtyar, M. , & Haley, A. P. (2020). Cognition, brain structure, and brain function in individuals with obesity and related disorders. Current Obesity Reports, 9, 544–549. 10.1007/s13679-020-00412-y [DOI] [PubMed] [Google Scholar]
- ten Kulve, J. S. , van Bloemendaal, L. , Balesar, R. , IJzerman, R. G. , Swaab, D. F. , Diamant, M. , la Fleur, S. E. , & Alkemade, A. (2016). Decreased hypothalamic glucagon‐like peptide‐1 receptor expression in type 2 diabetes patients. The Journal of Clinical Endocrinology and Metabolism, 101, 2122–2129. 10.1210/jc.2015-3291 [DOI] [PubMed] [Google Scholar]
- Turrigiano, G. G. , & Nelson, S. B. (2004). Homeostatic plasticity in the developing nervous system. Nature Reviews. Neuroscience, 5, 97–107. 10.1038/nrn1327 [DOI] [PubMed] [Google Scholar]
- Vice, E. , Privette, J. D. , Hickner, R. C. , & Barakat, H. A. (2005). Ketone body metabolism in lean and obese women. Metabolism, 54, 1542–1545. 10.1016/j.metabol.2005.05.023 [DOI] [PubMed] [Google Scholar]
- Virathone, L. , Nguyen, B. N. , Dobson, F. , Carter, O. L. , & McKendrick, A. M. (2021). Exercise alone impacts short‐term adult visual neuroplasticity in a monocular deprivation paradigm. Journal of Vision, 21, 12. 10.1167/jov.21.11.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. X. , McGraw, P. , & Ledgeway, T. (2020). Short‐term monocular deprivation reduces inter‐ocular suppression of the deprived eye. Vision Research, 173, 29–40. 10.1016/j.visres.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Yudkoff, M. , Daikhin, Y. , Nissim, I. , Lazarow, A. , & Nissim, I. (2001). Brain amino acid metabolism and ketosis. Journal of Neuroscience Research, 66, 272–281. 10.1002/jnr.1221 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Y. , Proenca, R. , Maffei, M. , Barone, M. , Leopold, L. , & Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homolog. Nature, 372, 425–432. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- Zhou, J. W. , Baker, D. H. , Simard, M. , Saint‐Amour, D. , & Hess, R. F. (2015). Short‐term monocular patching boosts the patched eye's response in visual cortex. Restorative Neurology and Neuroscience, 33, 381–387. 10.3233/RNN-140472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. W. , Clavagnier, S. , & Hess, R. F. (2013). Short‐term monocular deprivation strengthens the patched eye's contribution to binocular combination. Journal of Vision, 13, 1–10. 10.1167/13.5.12 [DOI] [PubMed] [Google Scholar]
- Zhou, J. W. , Reynaud, A. , & Hess, R. F. (2014). Real‐time modulation of perceptual eye dominance in humans. Proceedings of the Royal Society B: Biological Sciences, 281, 20141717. 10.1098/rspb.2014.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. W. , Reynaud, A. , Kim, Y. J. , Mullen, K. T. , & Hess, R. F. (2017). Chromatic and achromatic monocular deprivation produce separable changes of eye dominance in adults. Proceedings of the Royal Society B: Biological Sciences, 284, 20171669. 10.1098/rspb.2017.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitron‐Emanuel, N. , & Ganel, T. (2018). Food deprivation reduces the susceptibility to size‐contrast illusions. Appetite, 128, 138–144. 10.1016/j.appet.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Zitron‐Emanuel, N. , & Ganel, T. (2020). Does food deprivation affect perceived size? Appetite, 155, 104829. 10.1016/j.appet.2020.104829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available at 10.5281/zenodo.7360737.


