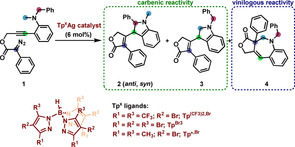
Table 1.
Initial screening of silver‐based catalysts for CAM‐cascade reactions.[a]
|
| ||
|---|---|---|
|
Entry |
Catalyst |
Yield [%] (2anti /2syn /3/4) |
|
1[b] |
Tp(CF3)2,BrAg(THF) |
45[d] (23/17/0/5) |
|
2 |
Tp(CF3)2,BrAg(THF) |
82[d] (39/22/0/21) |
|
3 |
[TpBr3Ag]2 |
95 (35/21/18/21) |
|
4[c] |
Tp*,BrAg(THF) |
39 (14/8/17/0) |
[a] Unless otherwise noted, reactions were carried out with 0.06 mmol of 1 ([1]=3.8 mM), at room temperature in 16 mL of DCM for 1.5 h. The yield was determined by NMR using 4‐chlorobenzaldehyde as internal standard. Product ratios determined by NMR. [b] Reaction carried out at [1]=20 mM for 1 h. [c] Reaction run for 18 h. [d] Isolated yield.