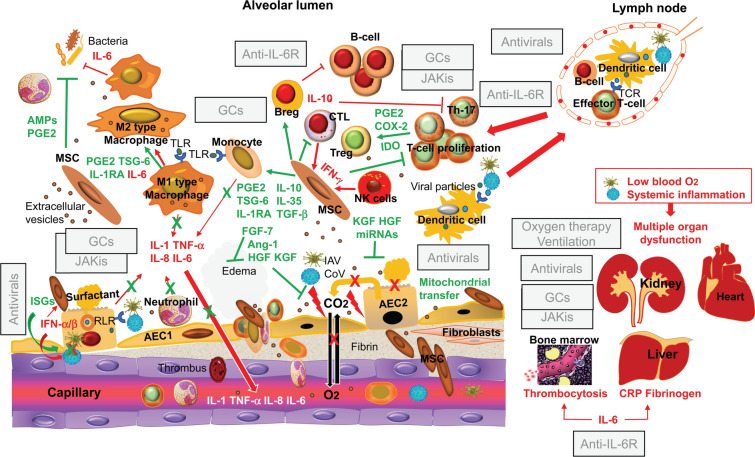
Fig. 2.
Mechanistic rationale for investigating the clinical use of mesenchymal stem/stromal cells and their products as adjunctive therapy for the management of severe and critical coronavirus disease 2019 and influenza. The pathological mechanisms leading to alveolar damage, hypoxemia, and systemic inflammation are highlighted in red, and the counteracting effects of intravenously injected allogeneic mesenchymal stem/stromal cells that have been induced to express an antiinflammatory/immunosuppressive phenotype systemically and in the inflamed alveoli are highlighted in green. In severe and critical coronavirus disease 2019, virus replication, the proliferation of effector T lymphocytes, the release of proinflammatory cytokines, and the recruitment of leukocytes from the peripheral blood are inhibited by the recommended treatment with antivirals and the glucocorticoid dexamethasone in combination with a Janus kinase inhibitor of the JAK1/JAK2 subtype, such as baricitinib, or with the humanized antibody against the interleukin-6 receptor tocilizumab. This combination also reduces the systemic effects of viral replication and excessive inflammation, but in abolishing the acute-phase response and the IL-6 mediated enhancement of bacterial phagocytosis, the combination of antiinflammatory agents concurs to render the host more vulnerable to pulmonary and systemic infections. Key adjunctive effects of mesenchymal stem/stromal cells are the following: reestablishment of the regulatory function of subpopulations of T and B lymphocytes (Treg and Breg cells) that normally suppress excessive and deleterious immunological/inflammatory responses; activation of the mechanisms involved in the repair of the alveolar-capillary barrier via the release of soluble factors (Ang-1, HGF, and KGF) and extracellular vesicles delivering microRNAs; enhancement of the viability of alveolar epithelial cells through the transfer of healthy mitochondria by intercellular communication; prevention of the development of secondary bacterial infections by producing antimicrobial peptides and by enhancing the phagocytic activity of neutrophils and macrophages through the release of prostaglandin E2. In severe and critical influenza, where a combination of antivirals and antiinflammatory or immunoregulators is not allowed, most of the biological effects of mesenchymal stem/stromal cells highlighted in this figure would be desirable. Generated using in part ScienceSlides graphics from VisiScience Corp., licensed use. AEC alveolar epithelial cell, AMPs antimicrobial peptides, Ang angiopoietin, Breg B regulatory lymphocytes, CRP C-reactive protein, CoV coronavirus, COX cyclooxygenase, CTL cytotoxic T lymphocytes, EVs extracellular vesicles, FGF fibroblast growth factor, GCs glucocorticoids, HGF hepatocyte growth factor, IAV influenza virus, IDO indoleamine 2,3-dioxygenase, IFN interferon, IL interleukin, IL1-RA interleukin 1 receptor antagonist, IL-6R interleukin-6 receptor, ISGs interferon-stimulated genes, JAKis Janus kinase inhibitors, KGF keratinocyte growth factor, miRNA microRNA, NK natural killer, PGE2 prostaglandin 2, RLR retinoic acid-inducible gene-1-like receptor, TCR T cell receptor, TGF transforming growth factor, Th T helper lymphocyte, TLR toll-like receptor, TNF tumor necrosis factor, Treg T regulatory lymphocyte, TSG tumor necrosis factor-stimulated gene