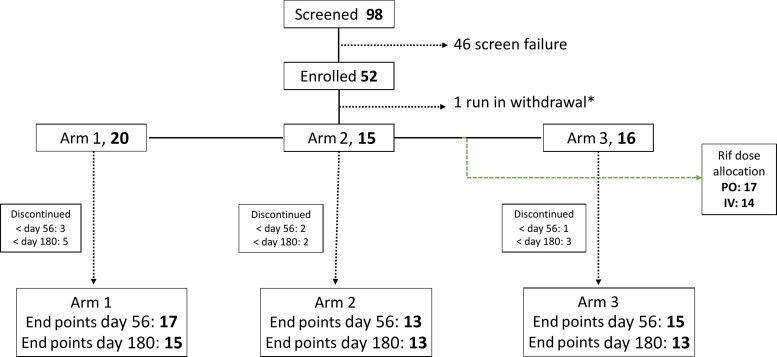
Figure 1.
Consolidated Standards of Reporting Trials diagram describing recruitment and arm allocation. One participant was randomized but excluded prior to any study investigational product (IP) being dispensed due to emergence of an exclusion criterion (estimated glomerular filtration rate <20) on a hospital blood test performed prior to randomization. Another participant was excluded from the modified intention-to-treat analysis as they died prior to receiving any dose of study drug. Six participants discontinued the study prior to day 56, and an additional 4 participants discontinued between day 56 and day 180 (Supplementary Table 2). Reasons for screening exclusions and early study withdrawals are listed in Supplementary Tables 1 and 2. *Patient randomized but withdrawn prior to receiving study IP due to emergence of exclusion criteria. Abbreviations: IV, intravenous; PO, by mouth; Rif, rifampicin.