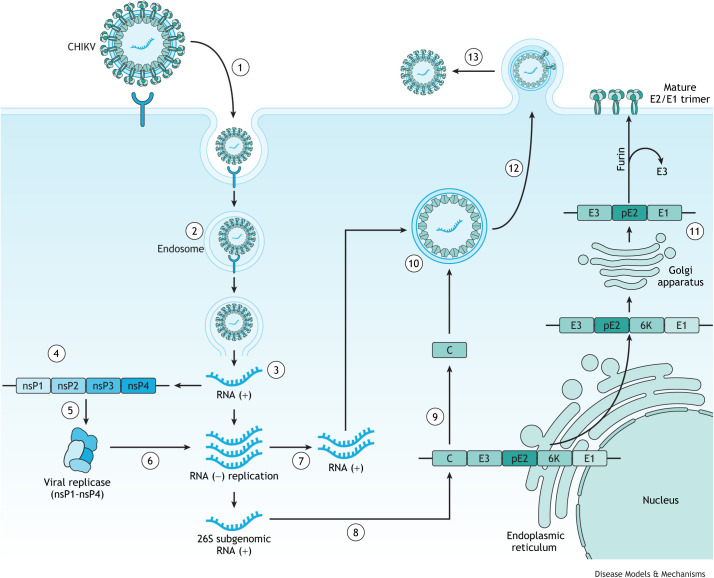
Fig. 1.
CHIKV life cycle. (1) CHIKV enters the host cells through receptor-mediated endocytosis. The viral protein E2 interacts with specific host surface receptors. A number of receptors [for example, prohibitin (Wintachai et al., 2012), MXRA8 (Zhang et al., 2018), PS receptors (Moller-Tank et al., 2013) and GAG (Silva et al., 2014)] have been implicated in this process. (2) Once in the endosome, the acidic pH triggers conformational changes in the viral envelope, exposing the E1 peptide and leading to its fusion with the endosomal membrane (Voss et al., 2010). (3) This allows for the cytoplasmic delivery of the nucleocapsid and the release of the viral RNA genome. (4) The viral genome is translated to the non-structural polyprotein nsP1- nsP2-nsP3-nsP4. (5) The viral protease nsP2 then cleaves the polyprotein into the individual nsPs – nsP1, nsP2, nsP3 and nsP4 – that then form the viral replicase complex. (6,7) The viral replicase is responsible for the synthesis of the negative-strand RNA that will be a template for both new positive-strand RNA and the sub-genomic RNA (26S RNA) (van der Heijden and Bol, 2002). (8) The 26S RNA drives the expression of the structural polyprotein C-pE2-6K-E1 in the endoplasmic reticulum. (9,10) The capsid protein (C) dissociates from the polyprotein by self-cleavage activity and binds to the newly synthesised viral RNA, forming the nucleocapsid core in the cytoplasm. (11) In the meantime, E2 and E1 associate in the Golgi apparatus and are exported to the cell membrane, where pE2 is cleaved by the host protease furin into E2 and E3. E3, which stabilises the E2/E1 trimer, then dissociates from the trimer when it reaches the cell membrane. (12) The already-formed nucleocapsid migrates to the host cell membrane region rich in E2/E1 trimers that bind the virion membrane. (13) The mature virions are released by the budding process from the infected cells (Yap et al., 2017). CHIKV, chikungunya virus; GAG, glycosaminoglycan; MXRA8, matrix remodelling-associated protein 8; nsP, non-structural protein; PS receptor, phosphatidylserine-mediated entry-enhancing receptor.