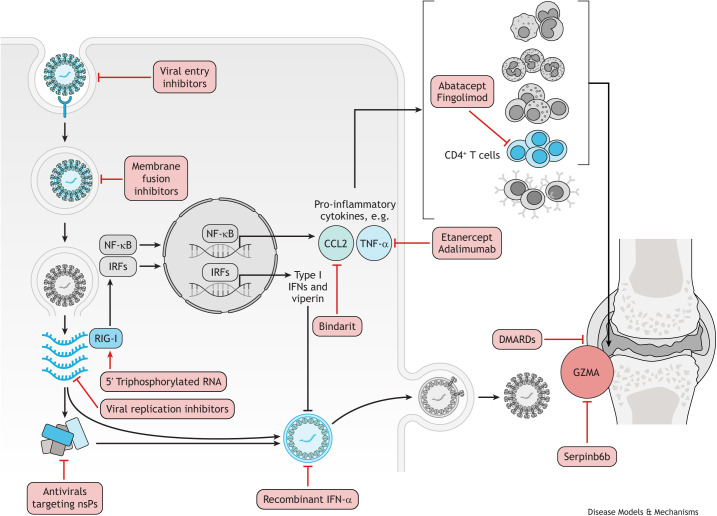
Fig. 3.
CHIKV treatments targeting the viral life cycle, host defence mechanisms and host immunopathology. Antivirals that block viral entry into the host cell include the natural compounds epigallocatechin gallate (Weber et al., 2015), flavaglines (Wintachai et al., 2015) and curcumin (Mounce et al., 2017). In vitro, chloroquine was shown to increase the endosomal pH, which then prevents the fusion of CHIKV E1 glycoprotein with the endosomal membrane (Khan et al., 2010). Broad-spectrum antivirals, including ribavirin and 6-azauridine, inhibit viral replication by introducing mutations in the viral genome, whereas favipravir targets RNA polymerase and prevents viral replication (Briolant et al., 2004). The viral nsPs are also a potential target for the development of antiviral molecules, and several compounds have been identified to target different nsPs, including harringtonine (Bassetto et al., 2013; Das et al., 2016). In response to CHIKV infection, the host elicits a strong antiviral type I IFN; therefore, recombinant IFN-α has shown the ability to inhibit CHIKV replication in vitro (Briolant et al., 2004). Furthermore, activating the RIG-I receptor with 5′ triphosphorylated RNA can augment this host response (Olagnier et al., 2014). A prolonged pro-inflammatory host response to CHIKV infection can lead to the chronic disease; therefore, some treatments are aimed at dampening this response. Inhibiting the chemokine CCL2 with bindarit ameliorated the inflammation in joints and skeletal muscles in mice (Rulli et al., 2011). TNF-α inhibitors, such as etanercept and adamimumab, were shown to reduce arthritic symptoms in patients (Blettery et al., 2016). CD4+ T cells also have a primary role in the pathogenesis of CHIKV-induced joint inflammation (Teo et al., 2017). Fingolimod efficiently suppressed CHIKV-induced joint pathology in virus-infected mice by blocking T-cell migration from the lymph nodes to the joints (Teo et al., 2017), and abatacept, which inhibits CD4+ T-cell priming in combination with neutralising anti-CHIKV monoclonal antibody, reduced T-cell accumulation in the joints (Miner et al., 2017). This resulted in ameliorated joint inflammation and decrease in proinflammatory cytokine secretion (Miner et al., 2017). The pro-inflammatory granule GZMA is released by natural killer cells and CD8+ T cells in response to CHIKV infection, and the GZMA inhibitor, Serpinb6b, was shown to reduce joint inflammation (Wilson et al., 2017). DMARDs, including hydroxychloroquine, sulfasalazine and methotrexate, have been trialled for chronic CHIKV arthritis (Ganu and Ganu, 2011; Martí-Carvajal et al., 2017; Ravindran and Alias, 2017). However, owing to contradictory results, it remains unclear whether the use of specific DMARDs is effective in treating chronic CHIKV arthritis. CHIKV, chikungunya virus; DMARD, disease-modifying antirheumatic drug; GZMA, granzyme A; IFN, interferon; IRF, interferon regulatory factor; NF-κB, nuclear factor kappa B; nsP, non-structural protein; RIG-I, retinoic acid-inducible gene I; TNF-α, tumour necrosis factor alpha.