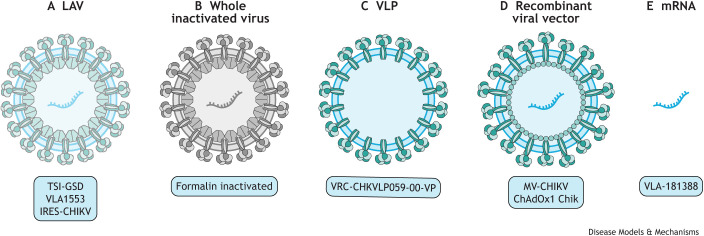
Fig. 4.
Several platforms used for the development of CHIKV vaccines that have entered human clinical trial testing. (A) LAV vaccines are derived from CHIKV, with structural changes introduced to reduce its virulence but retain its immunogenicity. The CHIKV LAV vaccines that have reached human clinical trials include TSI-GSD (The Salk Institute-Government Services Division), which showed promising results in phase I and II trials, but, owing to limited funding and uncertainty about safety, the development of this vaccine was terminated (Edelman et al., 2000); the Δ5nsP3 vaccine (VLA1553), which recently entered phase III clinical trial (NCT04546724); and IRES-CHIKV, which is in its late preclinical stages, in which immunogenicity and efficacy have been tested in mice and non-human primates (Plante et al., 2011; 2015). (B) The use of whole inactivated virus via chemical treatment, with formalin, was one of the first strategies for the development of a vaccine for CHIKV. The formalin-inactivated CHIKV vaccine entered clinical phase I (Harrison et al., 1971); however, despite the good safety, tolerability and immunogenicity profiles, the vaccine programme was discontinued. (C) VLP vaccines closely resemble the wild-type virus by containing self-assembled structural proteins but no viral genetic material. A CHIKV VLP vaccine candidate, VRC-CHKVLP059-00-VP, has now advanced into clinical phase II evaluation (NCT02562482) (Akahata et al., 2010). (D) Chimeric or recombinant viral-vectored vaccines are obtained by incorporating genetic elements of CHIKV into a vector virus genome. The recombinant viral-vectored vaccine MV-CHIKV, which uses attenuated measles virus as a vector, is currently in clinical phase II (NCT02861586), and ChAdOx1 Chik vaccine, known as the chimpanzee adenovirus Oxford 1 as it uses the Simian adenovirus as a vector, is in phase I of a clinical trial (NCT03590392). (E) mRNA vaccines are the newest strategy for the development of CHIKV vaccine candidates, based on the delivery of engineered mRNAs that can instruct host cells to express viral antigens, mimicking a viral infection that could elicit an immune response and lead to the generation of antibodies. There is currently one CHIKV mRNA vaccine, VLA-181388, in phase I clinical trial (NCT03325075) (Goyal et al., 2018). CHIKV, chikungunya virus; LAV, live-attenuated virus; VLP, virus-like particle.