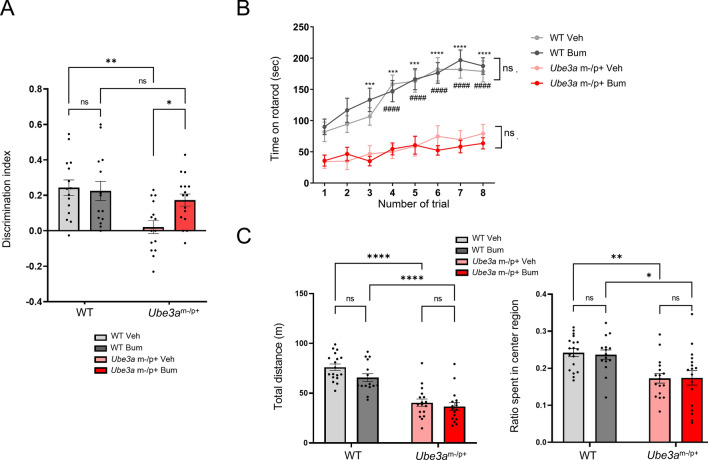
Figure 3.
Effects of chronic bumetanide administration on behavioral test results in Ube3am−/p+ mice. (A) Effects of bumetanide on the results of the novel object recognition test. ns not significant, *p < 0.05; **p < 0.01 by post-hoc analysis after two-way ANOVA. n = 14–16 for each group. (B) Effects of bumetanide on the performance of mice in the accelerating rotarod test. ns notsignificant between treatment with bumetanide (Bum) and vehicle control (Veh). ***p < 0.001; ****p < 0.0001 for wild-type (WT) Veh versus Ube3am−/p+ Veh. ####: p < 0.0001 for WT Bum versus Ube3am−/p+ Bum by post-hoc analysis after three-way repeated-measures ANOVA. n = 15–18 for each group. (C) Effects of bumetanide on the results in the open field test. The left and right panels show the total distance traveled and ratio time spent in the center region, respectively. ns not significant. *p < 0.05; **p < 0.01; ****p < 0.0001 by post-hoc analysis after two-way ANOVA. n = 15–18 for each group. The open field and rotarod test results are not affected by bumetanide, but bumetanide restores the low discrimination index of Ube3am−/p+ mice in the novel object recognition test.