FIGURE 10.
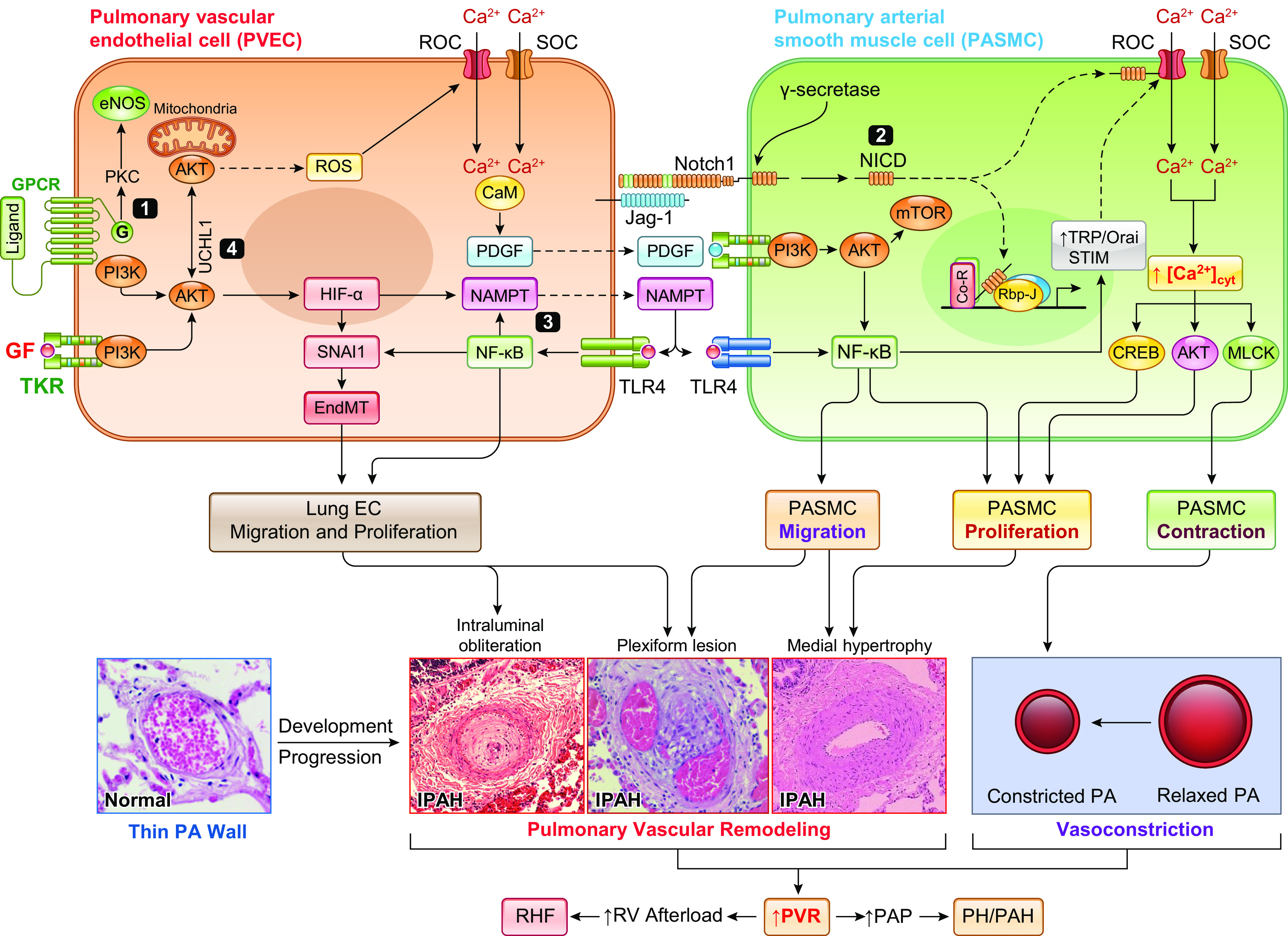
Potential pathogenic role of paracrine and juxtacrine interactions between pulmonary vascular endothelial cells (PAECs) and smooth muscle cells (PASMCs) in the development of pulmonary hypertension (PH). Aberrant phosphorylation of endothelial nitric oxide synthase (eNOS) due to G protein-coupled receptor (GPCR)-mediated activation of protein kinase C (PKC) results in eNOS uncoupling and reactive oxygen species (ROS) generation leading to increased EC proliferation via an eNOS-Akt-mitochondrial hypoxia-inducible factor (HIF) signaling axis (345, 346). The phosphatidylinositol 3-kinase (PI3K)/Akt/HIF/SNAI1 signal axis or the constitutively upregulated HIF-2α due to reduced PHD2 (63, 348, 349) results in endothelium-to-mesenchymal transition (EndMT), upregulates Notch ligand Jag-1, and increases the synthesis and production of platelet-derived growth factor (PDGF) and nicotinamide phosphoribosyltransferase (NAMPT) in PAECs. EndMT converts slow-growing PAECs to highly proliferative myofibroblasts (myoFBs), causing obliterative intimal lesions. Juxtacrine activation of Notch signaling via PAEC-PASMC interaction functionally activates and transcriptionally upregulates receptor-operated Ca2+ channels (ROCs) and store-operated Ca2+ channels (SOCs) to increase cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMCs, leading to PASMC contraction and pulmonary vasoconstriction and to PASMC proliferation and concentric pulmonary medial hypertrophy. HIF-mediated upregulation of PDGF and NAMPT (254, 255) in PAECs activates their receptors [PDGF receptor (PDGFR) and Toll-like receptor 4 (TLR4), respectively] in PASMCs through a paracrine mechanism, which results in PAMSC migration and proliferation through the PI3K/Akt/mammalian target of rapamycin (mTOR) (350–354) and NF-κB (123, 355) signaling pathways. Increased PASMC migration and proliferation contribute to the development and progression of concentric pulmonary vascular remodeling, arteriole muscularization, and occlusive vascular lesions. Enhanced NAMPT also activates TLR4/NF-κB signaling in PAECs via autocrine mechanism to enhance inflammation-associated PAEC proliferation and migration, whereas UCHL1-mediated regulation of AKT degradation further contributes to enhancing PAEC proliferation by promoting HIF and NF-κB signaling cascades (356, 357). Both NAMPT-NF-κB and Akt1/mTOR signaling also upregulate ROCs and SOCs involved in receptor- and store-operated Ca2+ entry, increase [Ca2+]cyt, and further induce PASMC contraction, migration, and proliferation. Ultimately, concentric PA wall thickening, sustained pulmonary vasoconstriction, and obliterative lung vascular lesions all contribute to increasing pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) in patients with pulmonary arterial hypertension (PAH). IPAH, idiopathic PAH; MCLK, myosin light chain kinase; NICD, Notch intracellular domain; RHF, right heart failure; TKR, tyrosine kinase receptor.
