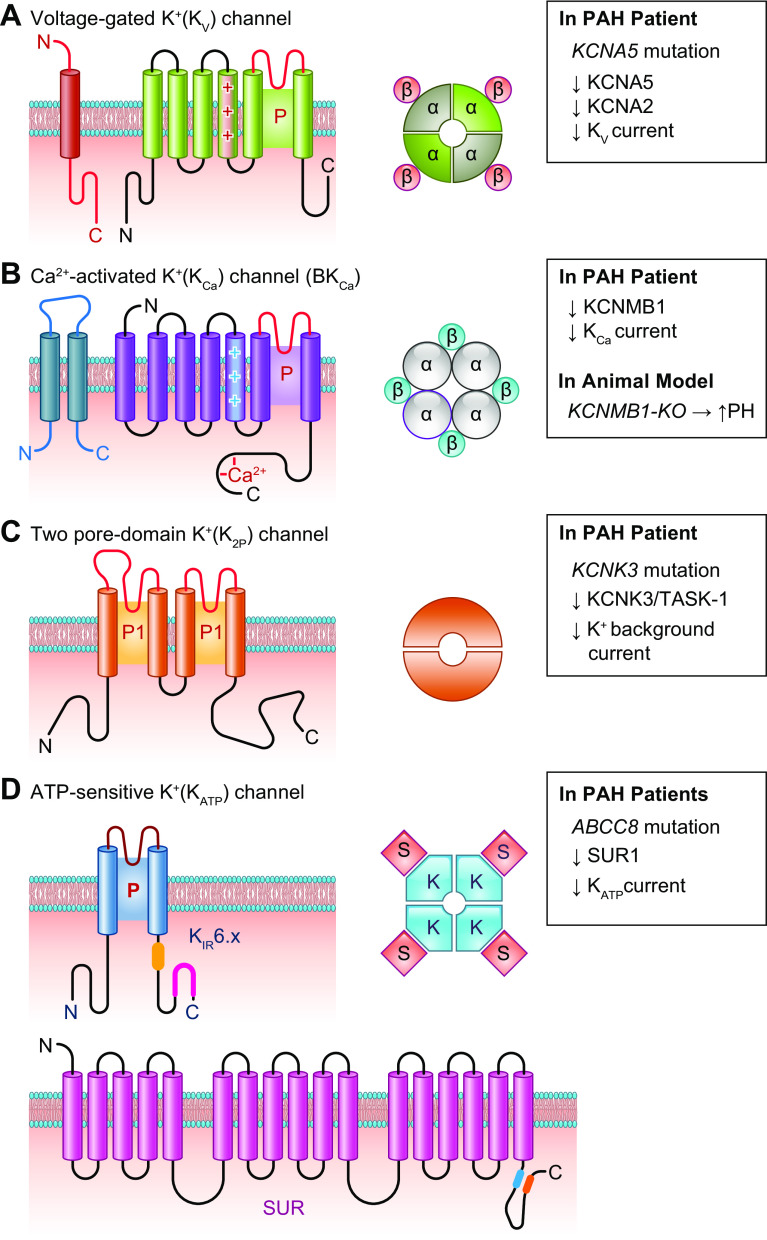
FIGURE 17.
Schematic diagram showing structure of K+ channels. Planar membrane topologies of single K+ channel subunits for a voltage-gated (KV) K+ channel (A), a Ca2+-activated (BKCa) K+ channel (B), a 2-pore domain (K2P) K+ channel (C), and a ATP-sensitive K+ (KATP) channel (D), which is composed of inward-rectifier (KIR) K+ channel and sulfonylurea receptor (SUR). The pore-forming loop is indicated (P) and the voltage sensor (+) in the fourth transmembrane domain (TMD4) for KV (A) and BKCa (B) channels, which are homotetramers or heterotetramers with 4 β-subunits (left and center). C: membrane topology of a K2P channel subunit featuring 2 pore regions, P1 and P2, and 4 transmembrane spanning domains and cytoplasmic NH2 and COOH termini. The functional K2P channels are thus dimers. D: the KATP channels are heterooctamers formed by 4 pore-forming KIR subunits (e.g., KIR6.x) and 4 SURs. Right: the channel genes associated with pulmonary arterial hypertension (PAH) and the decreased (↓) whole cell K+ currents through different K+ channels found in PASMCs from PAH patients or related to the mutations/single-nucleotide polymorphisms (SNPs) identified from PAH patients. For BKCa channels, knockout (KO) of KCNMB1, a β-subunit of the large-conductance KCa channel, enhances experimental pulmonary hypertension (PH) in mice.