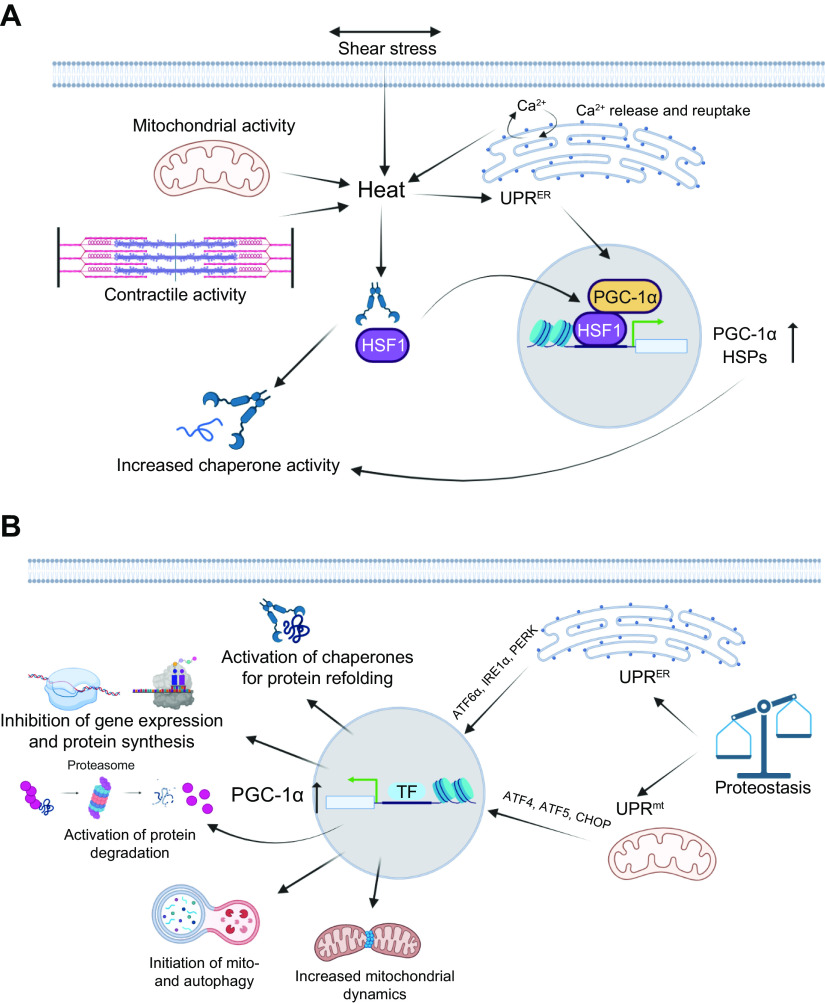
FIGURE 15.
Heat and proteostatic stress. A: thermosensing and the heat stress response mitigate misfolding of proteins. Heat is produced by various processes in the contracting muscle fiber, including shear stress, mitochondrial activity, Ca2+ release and reuptake, and ATP metabolism in contraction cycling. Heat is sensed in the cell and a broad transcriptional program engaged to increase mitigating measures, e.g., chaperones to reduce thermally induced protein misfolding. B: proteostatic stress and the ensuing response pathways reduce protein load, misfolding, and organelle health. Dedicated pathways in the endoplasmic reticulum and mitochondria are engaged by proteostatic dysbalances, e.g., excessive protein accumulation or misfolding. At least in part, these two pathways converge to initiate a transcriptional program aimed at reversing protein misfolding, alleviating proteostatic stress by reducing gene expression and protein synthesis while enhancing protein degradation, and by ensuring organelle functionality. ATF4/5/6α, activating transcription factor 4/5/6α; CHOP, C/EBP homologous protein; HSF1, heat shock factor 1; HSP, heat shock protein; IRE1α, inositol-requiring enzyme 1α; PERK, RNA-dependent protein kinase-like ER eukaryotic translation initiation factor 2α kinase; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; TF, transcription factor; UPRER, endoplasmatic reticulum unfolded protein response; UPRmt, mitochondrial unfolded protein response. Image created with BioRender.com, with permission.