Cryptococcosis is a prominent cause of fungal meningoencephalitis with a significant disease burden globally1. Although this infection is more often an opportunistic consequence of HIV/AIDS infection, it has also been reported in apparently immunocompetent populations.2 Currently, it is reported that approximately 20% of cases occur in clinically non-immunocompromised patients 3,4 and the disease course in these patients has been associated with poorer CSF inflammatory responses and mortality rates.5
CM most frequently manifests in patients as headaches, fever, nausea, vomiting, and visual impairment. When the eye is involved, patients may experience photophobia, diplopia, ptosis, nystagmus and ophthalmoplegia.6 Isolated ocular cryptococcus with no neurological involvement has been reported.7 In previously healthy adults with CM, ocular manifestations include optic neuropathy, cranial nerve VI palsies, chorioretinitis, vitritis, posterior uveitis and papilledema.8,9
While there are several case reports in the literature describing ocular findings in previously healthy CM patients, rare reports exist depicting these findings in a substantial cohort. In the present study of 44 previously healthy patients with CM, we identify abnormal ocular exam findings, attempt to detect an association with key CSF markers and MRI findings.
METHODS
Ethics Statement
All patients were seen at the National Institutes of Health Clinical Center, Bethesda, MD, and informed consent was obtained under an institutional review board-approved protocol (ID: 93-I-0106).
Participants and Patient Clinical Data
To characterize the type and severity of vision health in CM, a retrospective study was performed involving previously healthy patients with CM who underwent a comprehensive ophthalmological exam between January 2010 and August 2016 at the NIH clinical center. A diagnosis of CM was defined as a positive cryptococcal antigen in CSF and/or the isolation of Cryptococcus from CSF cultures. All participants in this study were diagnosed with CM at another facility and subsequently referred to the NIH for immuno-genetic testing or due to deterioration in clinical status after receiving anti-fungals. Prior to arrival at NIH, all patients had received standard induction therapy with parenteral Amphotericin B and flucytosine for at least 4 weeks or 2 weeks after CSF fungal cultures became negative. Patients were excluded if they were HIV positive, transplant recipients, on immunosuppressants or recently diagnosed with a malignancy. Available cryptococcal isolates were shipped to the NIH and speciated as part of the protocol.
Demographic and clinical data including symptoms, results of CSF analysis and radiological impressions on MRI brain were recorded upon admission to the NIH. MRI findings were assessed in a blinded fashion to visual loss, focusing on ventriculomegaly, hydrocephalus and meningeal enhancement on post-gadolinium fluid-attenuated inversion recovery (FLAIR) images. Details of the initial ophthalmologic evaluation were noted; of interest were visual acuity (VA), presence of an afferent pupillary defect (APD), color vision deficits, applanation tonometry (IOP), cup to disc ratio (C/D), grading of disc edema according to the Frisen scale, optical coherence tomography retinal nerve fiber layer (RNFL) thickness, and Humphrey Visual Field (HVF) 30-2. Ocular Coherence Tomography (OCT) RNFL thickness was obtained for patients who presented after 2014 when it became part of the initial exam. Among the variables assessed on initial ophthalmological exam, data were missing for ~30% of the patients for the Ishihara test (color vision), presence of cataract and degree of papilledema and these parameters were therefore excluded for analysis. In patients who presented with altered mental status, a complete ophthalmological exam could not be performed at the time of presentation and was completed at a later date after clinical improvement.
The best corrected visual acuity was measured for both eyes using the Snellen chart and pinhole vision. RNFL thickness was measured via Cirrus-OCT and considered normal for values ranging from 75-115 microns. HVF result was categorized as normal if the mean deviation (MD) was </= −2 and abnormal if greater than this value. Snellen visual acuity was converted to LogMAR vision for statistical analysis, vision below 20/40 was considered decreased vision.
Statistical Analysis
Data was stored and analyzed on the SAS version 9.4 software, R version 4.0.2.and GraphPad prism version 8.0.
Left and right ocular variables that are continuous, were summarized in terms of mean or geometric mean10. Median values were computed for RNFL, HVF mean deviation, tonometry, Ishihara color testing, and cup to disc ratio; geometric mean was computed for pinhole and Snellen vision results after conversion to logMAR vision. Associations between categorical variables were evaluated with either the Chi-square test or Fisher’s exact test; associations of categorical variables with continuous variables were evaluated using the Wilcoxon rank-sum test; correlations between continuous variables were assessed using Spearman rank correlation coefficients.
RESULTS
Study Subjects
Forty-four patients admitted with cryptococcal meningitis between January 2010 and August 2016 who had comprehensive ophthalmological exams were included in this study; 15 (34%) were female. Five patients (11%) had an underlying history of diabetes and 22 (50%) had hypertension; there was no known history of cirrhosis in any patient. Importantly, 31 patients (70%) had not been shunted at the time their eye exam was performed. Additional demographic details are presented in Table 1.
Table 1.
Demographic details, symptoms at presentation and CSF parameters for all patients who underwent an eye exam
| Demographic characteristics | N=44 (%) |
|---|---|
| Gender, females | 15 (34) |
| Median Age in years at first eye exam (IQR) | 56 (44-64) |
| Ethnicity: Asian | 4 (9) |
| African American | 2 (4.5) |
| Caucasian | 35 (80) |
| Hispanic | 2 (4.5) |
| Biracial | 1 (2) |
| Location Midwest US | 1 (2) |
| NE US | 16(36) |
| SE US | 17 (39) |
| W Coast US | 10(23) |
| Species (n=39) | |
| Neoformans | 29 (74%) |
| Gattii | 10 (26%) |
| GMCSF auto Ab (n=34) | 6(18%) |
| Median CD 4 count (IQR) | 452 (304-769) |
| Underlying conditions | |
| Diabetes | 5(11) |
| Hypertension | 22(50) |
| No. shunted at the time of eye exam | 13 |
| Time in weeks from CM diagnosis to eye exam (IQR) | 12 (5-21) |
| Disease presentation | |
| Fever | 13(30) |
| Headache | 40 (91%) |
| Worsening mental status | 16 (36%) |
| Hearing loss | 21 (48%) |
| Vision change | 27 (61%) |
| Nausea and vomiting | 25 (57%) |
| Falls | 4 (9%) |
| Dizziness | 11(25%) |
| Gait instability | 9 (20%) |
| CSF parameters | Median (IQR) |
| Cryptococcal antigen titer (n=44) | 1:64(1:1-1:256) |
| Opening pressure (cm CSF) (n=43) | 20 (15-28) |
| Glucose (mg/dL) (n=44) | 47(29-56) |
| Protein (mg/dL) (n=43) | 84 (57-199) |
| WBC (/mm3) (n=42) | 39(14-91) |
Clinical findings
The most common symptoms at CM diagnosis included headache (91%), vision change (61%), hearing loss (48%), nausea and vomiting (57%) (Table 1). Upon transfer to NIH, a lumbar puncture (LP) was performed within a median of 9 weeks (IQR 4-25) after CM diagnosis. The median opening pressure was 20 cm CSF (IQR 15-28, n=43) and the median CSF cryptococcal antigen titer was1:64 (IQR 1:1 −1:256;Table 1). MRI brain scans were performed for all patients at a median of 12 weeks from diagnosis (IQR 6-22), results of which showed leptomeningeal enhancement in 32 (73%), ventriculomegaly in 19 (43%) and hydrocephalus in 6 (14%) of patients.
Ocular findings:
Each patient received a complete, dilated eye exam within a median of 12 weeks (IQR 5-21) after CM diagnosis. Data describing ocular findings is summarized in Table 2. The median RNFL thickness was 98 microns ( IQR 86-141, n=31) with 8 (26%) patient measurements being >115μm, suggestive of papilledema and 2 (6%) patients having a measurement of <75μm, indicating optic nerve atrophy. Five of 8 patients with papilledema had an elevated opening pressure (>25 cm H2O) on initial LP.
Table 2.
Ocular findings in previously healthy adults with CM.
| Ocular measurements | Number/% tested | Median (IQR) | Normal range |
|---|---|---|---|
| RNFL thickness (μm) | 31 (70) | 98 (86-141) | 75-115 |
| Visual field deficit (mean deviation in dB) | 32 (73) | −5.2 (−1 to −12) | 0 to −2 |
| Pinhole Visual acuity (LogMAR) | 30 (68) | 0.85*(0.63-1) | |
| Visual acuity (LogMAR) | 37 (84) | 0.63*(0.28-0.8) | |
| Intraocular pressure (mm Hg) | 41 (93) | 14 (12-16) | 10-23 |
| Cup to disk ratio | 35 (80) | 0.3 (0.1-0.3) | <0.5 |
Abbreviations: dB = decibels, LogMAR: Logarithm of the Minimum Angle of Resolution
Geometric median calculated for visual acuity
The median mean deviation (MD) on HVF testing was −5.2 dB (IQR −1.25 to −11.6, n=33) with 22 (67%) patients having a value of <−2 db. The most common HVF defects were superior (47%) followed by enlarged blind spots (22%), central scotomas (19%), heminanopsias (6%) and generalized depression (6%). HVF and RNFL thickness maps of a 27-year-old and 58-year-old patient with severe visual field defects and papilledema are shown in Figure 1 and 2 respectively. The geometric median calculated for best corrected visual acuity by logMAR vision was 0.6 (20/80 Snellen vision; IQR 0.28 - 0.8, n=37); 14 (38%) patients had a decreased visual acuity (<20/40).
Figure 1.
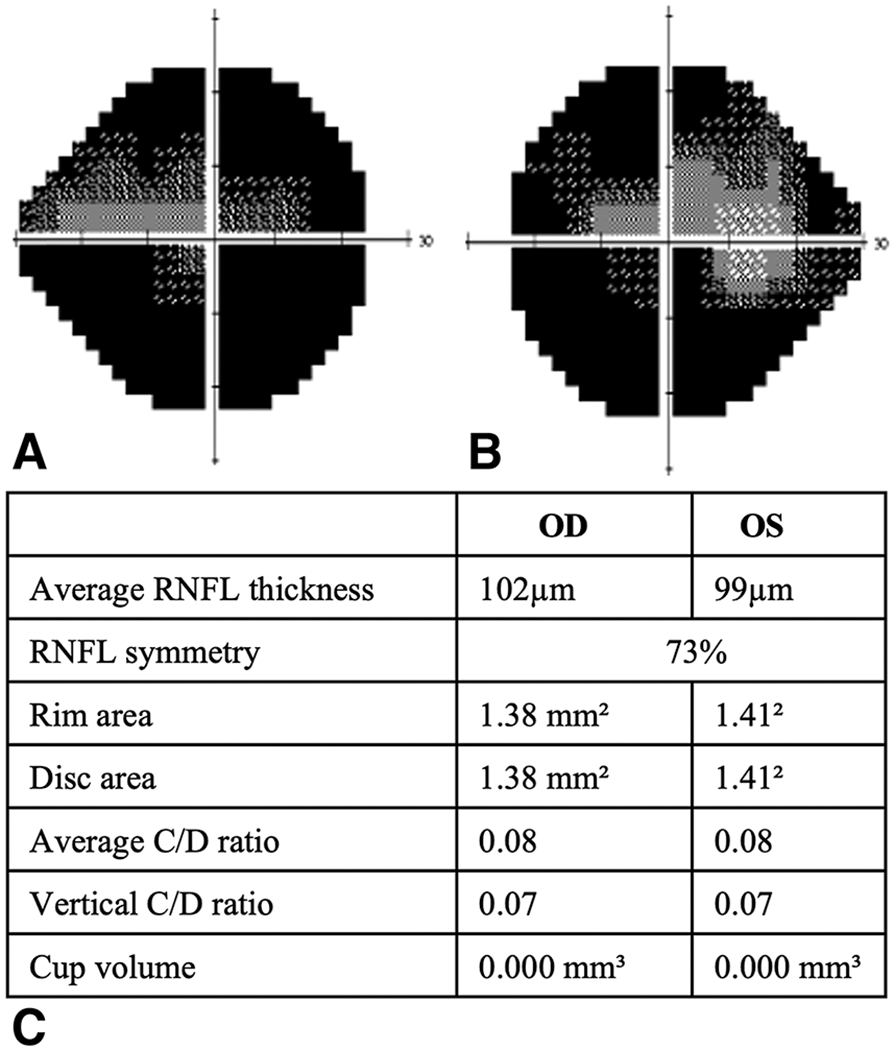
Right(A) and left (A) HVF, corresponding RNFL thickness map(C) for a 20v/o male with severe visual field defects and papilledema
RNFL: Retinal Nerve Fiber Layer
Figure 2.
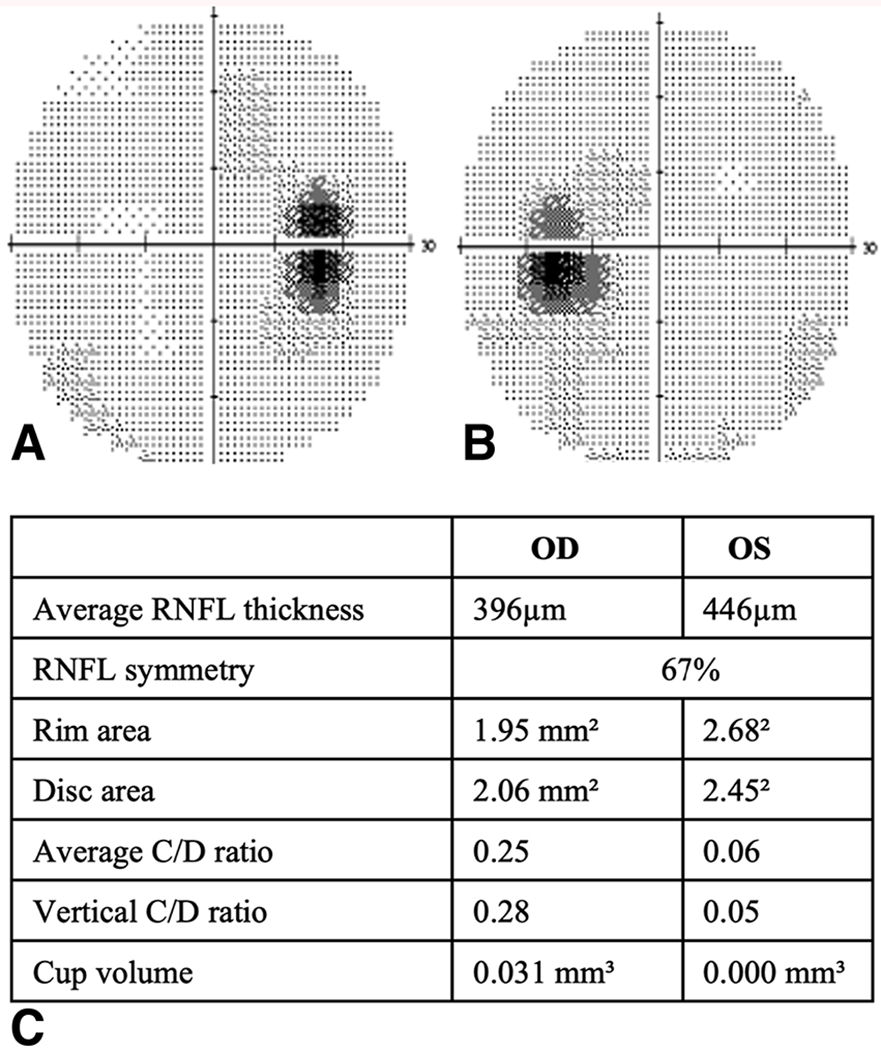
Right(A) and left (B) HVF, corresponding RNFL thickness map(C) for a 58y/o male with enlarged blind spots
The median intraocular pressure (IOP) was 14 mmHg(IQR 12-16, n=41) and all patients had an IOP within normal limits(<23 mmHg). All patients without papilledema in whom a cup to disc ratio was measured had values <0.5. The presence of an afferent pupillary defect was noted in 2 (6%) of the 31 patients who had a pupil exam; both patients had abnormal MD on HVF testing (−5 and −12 respectively), and one patient had a compromised visual acuity of 20/80. Cranial neuropathy was identified in five patients, of which 3 had cranial nerve VI palsy, 2 had cranial nerve III and IV palsy, respectively. Three patients had severe optic neuropathy (one unilateral and two bilateral) and presented with severely reduced visual acuity which was noted to be irreversible on subsequent exams. No retinal defects were identified.
Among the 44 participants, 13 (30%) underwent ventriculo-peritoneal (VP) shunt insertion prior to their first eye exam, 9 (20%) were shunted after their first exam and 22 (50%) did not receive a shunt. When CSF and ocular findings were compared between those that were shunted and not shunted, the median CSF protein was more elevated in the shunted population (226 mg/dL compared to 69 mg/dL in non-shunted patients, p=0.01), but there was no difference in ocular findings between both groups. In terms of radiographic abnormalities, patients who had hydrocephalus were not noted to have worse ocular abnormalities.
DISCUSSION
In the present study of 44 non-immunosuppressed patients with CM, we report for the first time OCT RNFL and HVF findings and their correlation to key CSF markers and radiographic findings in a sizeable cohort, which may help predict a poor prognosis in terms of vision. The most common ocular abnormalities noted were visual field defects in 21(66%), decreased visual acuity in 14 (38%) and papilledema in 8 (26%) patients. Intraocular pressure measured within a median of 12 weeks after CM diagnosis was within normal range in all patients. Cranial nerve defects were identified in 5 patients, and optic neuropathy in 2 patients. Overall, 29% of the population examined was shunted and there were no differences in ocular exam findings compared to the non-shunted group.
In a previous report of 82 previously healthy patients in Papua New Guinea diagnosed with CM and vision loss; 60% of cases were noted to have optic atrophy as a result of optic disc swelling.11 In another report, 31% of twenty-nine previously healthy patients with CM treated with standard antifungal therapy were blind at the time of discharge.12 On comparison of ocular findings in literature available for immunosuppressed patients, the disease course seems to be similar; in a retrospective study done in 18 Peruvian HIV-CM adults, half had decreased visual acuity and 2 had diplopia. In another case series, most had decreased visual acuity and diplopia, followed by papilledema and cranial nerve palsies. Abnormal pupillary reactions and microvasculopathy were rare. Another prospective study from South Africa reported early onset (within 2-4 weeks of CM diagnosis) decreased visual acuity in 46.5% patients and abnormal visual fields by HVF in 76% patients13, figures slightly higher than percentages found in our study. We also found fewer cases of optic atrophy (6%) and a low incidence of complete blindness in our study compared to prior literature.
In our cohort, 61% patients reported visual symptoms but the timeline for symptom progression is unknown since most patients were transferred from other referral centers approximately six weeks after the diagnosis was made. A previous study suggested an association between the timing of vision loss and the underlying mechanism. Patients with acute-onset vision loss were found to have optic neuropathy attributed to fungal infiltration of the optic nerve whereas those with gradual vision loss were found to have papilledema with slow loss of optic nerve axons.14 Twenty-six percent of patients in our study still had papilledema on presentation, suggestive of elevated intracranial pressure during the initial course of the disease as the most likely underlying mechanism for the observed findings. This further highlights the necessity of controlling intracranial pressures early in the course of the illness, a measure proven to both decrease mortality and prevent vision loss15.
Our results also demonstrated a positive correlation between the geometric mean of logMAR vision and visual field deficit Mean Deviation and a larger visual field defect correlated with poorer vision (figure 3, r=0.53, p>0.05), suggesting that in CM patients with decreased vision, a visual field defect is likely present as well.
Figure 3.
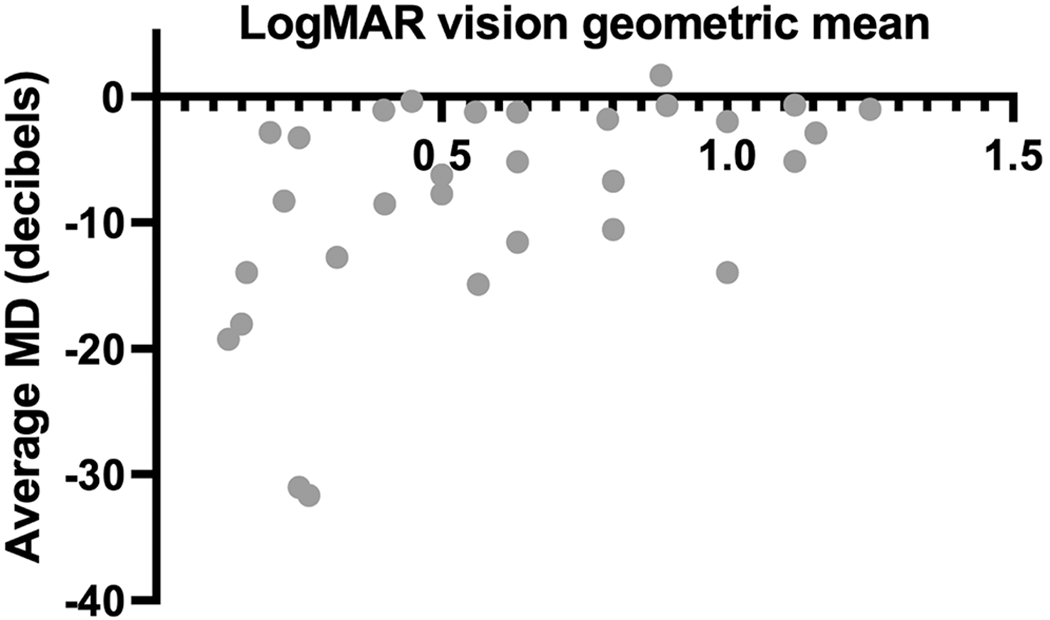
Scatter plot demonstrating a positive correlation between the geometric mean of logMAR vision and visual field deficit (1=0.53, p>0.05). Abbreviations: MD = mean deviation (HVF)
In the present study, it was difficult to confirm that the observed findings were due to the cryptococcal organism as opposed to an associated post-infectious inflammatory state due to the timing of the eye exams and limited data available regarding the timing of CSF fungal cultures converting to negative. Ophthalmological abnormalities in CM have been attributed to an excessive inflammatory response in both HIV and non-HIV hosts in earlier studies.16 Post-infectious inflammatory syndrome (PIIRS) in previously healthy adults is characterized by worsening visual acuity despite normal opening pressures and CSF fungal cultures turning negative with anti-fungal therapy, and is responsive to corticosteroids.17 Previously, we reported visual field defects and papilledema as the most common ocular defects in a case series of 15 previously healthy patients with CM and PIIRS.18
We did not find any correlation noted between elevated opening pressures (> 25 cm H2O) and increased RNFL thickness although 63% of patients with papilledema did have raised intracranial pressure. This is consistent with results noted by Graybill and colleagues in a cohort of 221 AIDS-associated CM patients in which papilledema was noted more frequently in those with an elevated intracranial pressure.19 The underlying mechanism for papilledema is the accumulation of CSF in the optic nerve sheath causing axoplasmic stasis in the optic nerve head leading to optic disc edema. The findings in the present study may have been due to the eye exam being performed at a median of 3 weeks after the lumbar puncture by which time intracranial pressures may have normalized in some patients. Additionally, exposure to papilledema for a prolonged period prior to the exam may have resulted in axonal loss and resultant decreased RNFL thickness in some patients. The lesson learned is that the absence of papilledema on ocular exam is not necessarily predictive of normal intracranial pressure, particularly in the acute setting.
In 6 (18 %) of our patients, the presence of GM-CSF auto antibodies in the serum was detected. Four had C. gatti isolated from CSF cultures, 1 had C. neoformans and for 1 patient, speciation data was not available. This corroborates findings reported previously which suggested that the presence of these antibodies may play a major role in compromised host immunity, specifically in response to C. gattii infection.20 However, we did not find any differences in eye findings within patients with or without GM-CSF auto antibodies. Auto antibodies to GM-CSF may suppress important immunological pathways mediated by pulmonary macrophages, phagocytes, and dendritic cells, thereby increasing host susceptibility to the fungus. Moreover, Cryptococcus independently is known to inhibit T cell-induced production of GM-CSF and TNF-α, a chemokine required for the maturation of dendritic cells which are crucial in recognition of the fungus once it enters the lungs.20
A limitation of this study is referral bias since most patients admitted to the NIH had refractory disease and the severity and frequency of ocular abnormalities may differ in the general population compared to that reported in our group. Additionally, the timing of the ocular exam (median of 12 weeks following CM diagnosis), does not exclude the possibility that visual defects may have been present earlier in the course of the disease. Finally, there are missing data for ocular parameters that could not be assessed at bedside for a few patients who presented with a significant alteration in their mental status, precluding a comprehensive eye exam.
In summary, our results emphasize the need for a comprehensive eye exam including visual field testing and OCT of the optic nerves in CM patients who may not always report a change in vision on presentation. Since papilledema may have detrimental effects on vision, it is imperative to control intracranial pressure along with concomitant antifungal therapy.
Funding:
This research was supported in part by the Intramural Research Program of the National Institutes of Health [AI001123 and AI001124] and by the Division of Intramural Research of the National Eye Institute.
Footnotes
Statement of Authorship
-Conception and design:
Chinwe Okeagu, Seher Anjum, Peter Williamson
-Acquisition of data:
Susan Vitale, Chinwe Okeagu, Seher Anjum, Deven Singh
-Analysis and interpretation of data:
Jin Wang, Seher Anjum
-Drafting the manuscript:
Chinwenwa Okeagu, Seher Anjum, Peter R. Williamson, Deven Singh, Teresa Magone, Edmond Fitzgibbon
-Revising the manuscript for intellectual content:
Teresa Magone, Edmond Fitzgibbon
-Final approval of the completed manuscript:
Teresa Magone, Edmond Fitzgibbon
Potential conflicts of interest: none
References
- 1.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. Aug 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 3.O’Halloran JA, Powderly WG, Spec A. Cryptococcosis today: It is not all about HIV infection. Curr Clin Microbiol Rep. Jun 2017;4(2):88–95. doi: 10.1007/s40588-017-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. Sep 1 2001;33(5):690–9. doi: 10.1086/322597 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MH, Husain S, Clancy CJ, et al. Outcomes of central nervous system cryptococcosis vary with host immune function: results from a multi-center, prospective study. J Infect. Nov 2010;61(5):419–26. doi: 10.1016/j.jinf.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Kestelyn P, Taelman H, Bogaerts J, et al. Ophthalmic manifestations of infections with Cryptococcus neoformans in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. Dec 15 1993;116(6):721–7. doi: 10.1016/s0002-9394(14)73472-5 [DOI] [PubMed] [Google Scholar]
- 7.Hester DE, Kylstra JA, Eifrig DE. Isolated ocular cryptococcosis in an immunocompetent patient. Ophthalmic Surg. Feb 1992;23(2):129–31. [PubMed] [Google Scholar]
- 8.Amphornphruet A, Silpa-Archa S, Preble JM, Foster CS. Endogenous Cryptococcal Endophthalmitis in Immunocompetent Host: Case Report and Review of Multimodal Imaging Findings and Treatment. Ocul Immunol Inflamm. 2018;26(4):518–522. doi: 10.1080/09273948.2017.1298820 [DOI] [PubMed] [Google Scholar]
- 9.Stone SP, Bendig J, Hakim J, Kinnear PE, Azadian BS, Clifford-Rose F. Cryptococcal meningitis presenting as uveitis. Br J Ophthalmol. Mar 1988;72(3):167–70. doi: 10.1136/bjo.72.3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. Jul-Aug 1997;13(4):388–91. [DOI] [PubMed] [Google Scholar]
- 11.Seaton RA, Verma N, Naraqi S, Wembri JP, Warrell DA. Visual loss in immunocompetent patients with Cryptococcus neoformans var. gattii meningitis. Trans R Soc Trop Med Hyg. Jan-Feb 1997;91(1):44–9. doi: 10.1016/s0035-9203(97)90391-6 [DOI] [PubMed] [Google Scholar]
- 12.Lalloo D, Fisher D, Naraqi S, et al. Cryptococcal meningitis (C. neoformans var. gattii) leading to blindness in previously healthy Melanesian adults in Papua New Guinea. Q J Med. Jun 1994;87(6):343–9. [PubMed] [Google Scholar]
- 13.Moodley A, Rae W, Bhigjee A, et al. Early clinical and subclinical visual evoked potential and Humphrey’s visual field defects in cryptococcal meningitis. PLoS One. 2012;7(12):e52895. doi: 10.1371/journal.pone.0052895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex JH, Larsen RA, Dismukes WE, Cloud GA, Bennett JE. Catastrophic visual loss due to Cryptococcus neoformans meningitis. Medicine (Baltimore). Jul 1993;72(4):207–24. doi: 10.1097/00005792-199307000-00001 [DOI] [PubMed] [Google Scholar]
- 15.Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. Dec 1 2014;59(11):1607–14. doi: 10.1093/cid/ciu596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana RN, Javaheri M, Rao N. Ophthalmic manifestations of immune reconstitution inflammatory syndrome associated with Cryptococcus neoformans. Ocul Immunol Inflamm. Jul-Aug 2008;16(4):185–90. doi: 10.1080/09273940802204550 [DOI] [PubMed] [Google Scholar]
- 17.Seaton RA, Verma N, Naraqi S, Wembri JP, Warrell DA. The effect of corticosteroids on visual loss in Cryptococcus neoformans var. gattii meningitis. Trans R Soc Trop Med Hyg. Jan-Feb 1997;91(1):50–2. doi: 10.1016/s0035-9203(97)90393-x [DOI] [PubMed] [Google Scholar]
- 18.Anjum S, Dean O, Kosa P, et al. Outcomes in Previously Healthy Cryptococcal Meningoencephalitis Patients treated with Pulse - Taper Corticosteroids for Post-infectious Inflammatory Syndrome. Clin Infect Dis. Dec 31 2020;doi: 10.1093/cid/ciaa1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. Jan 2000;30(1):47–54. doi: 10.1086/313603 [DOI] [PubMed] [Google Scholar]
- 20.Saijo T, Chen J, Chen SC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. Mar 18 2014;5(2):e00912–14. doi: 10.1128/mBio.00912-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


