Abstract
Background
Cumulative research show association of neutrophils and neutrophil extracellular traps (NETs) with poor outcomes in severe COVID-19. However, to date, there is no curative intent therapy able to block neutrophil/NETs-mediated progression of multi-organ dysfunction. Because of emerging neutrophil heterogeneity, the study of subsets of circulating NET-forming neutrophils [NET + Ns] as mediators of multi-organ failure progression among patients with COVID-19 is critical to identification of therapeutic targets.
Methods
We conducted a prospective observational study of circulating levels of CD11b + [NET + N] immunotyped for dual endothelin-1/signal peptide receptor (DEspR ±) expression by quantitative immunofluorescence-cytology and causal mediation analysis. In 36 consented adults hospitalized with mod-severe COVID-19, May to September 2020, we measured acute multi-organ failure via SOFA-scores and respiratory failure via SaO2/FiO2 (SF)-ratio at time points t1 (average 5.5 days from ICU/hospital admission) and t2 (the day before ICU-discharge or death), and ICU-free days at day28 (ICUFD). Circulating absolute neutrophil counts (ANC) and [NET + N] subset-specific counts were measured at t1. Spearman correlation and causal mediation analyses were conducted.
Results
Spearman correlation analyses showed correlations of t1-SOFA with t2-SOFA (rho rS = 0.80) and ICUFD (rS = -0.76); circulating DEspR + [NET + Ns] with t1-SOFA (rS = 0.71), t2-SOFA (rS = 0.62), and ICUFD (rS = -0.63), and ANC with t1-SOFA (rS = 0.71), and t2-SOFA (rS = 0.61).
Causal mediation analysis identified DEspR + [NET + Ns] as mediator of 44.1% [95% CI:16.5,110.6] of the causal path between t1-SOFA (exposure) and t2-SOFA (outcome), with 46.9% [15.8,124.6] eliminated when DEspR + [NET + Ns] were theoretically reduced to zero. Concordantly, DEspR + [NET + Ns] mediated 47.1% [22.0,72.3%] of the t1-SOFA to ICUFD causal path, with 51.1% [22.8,80.4%] eliminated if DEspR + [NET + Ns] were reduced to zero. In patients with t1-SOFA > 1, the indirect effect of a hypothetical treatment eliminating DEspR + [NET + Ns] projected a reduction of t2-SOFA by 0.98 [0.29,2.06] points and ICUFD by 3.0 [0.85,7.09] days. In contrast, there was no significant mediation of SF-ratio through DEspR + [NET + Ns], and no significant mediation of SOFA-score through ANC.
Conclusions
Despite equivalent correlations, DEspR + [NET + Ns], but not ANC, mediated progression of multi-organ failure in acute COVID-19, and its hypothetical reduction is projected to improve ICUFD. These translational findings warrant further studies of DEspR + [NET + Ns] as potential patient-stratifier and actionable therapeutic target for multi-organ failure in COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41231-023-00143-x.
Keywords: COVID-19, Multi-organ failure, NETs, Neutrophil-subsets, DEspR, Mediation analysis
Introduction
Activated neutrophils release neutrophil extracellular traps (NETs)—a DNA-weblike structure embedded with neutrophil microbicidal/cytotoxic proteases, enzymes, and decondensed histones – to entrap and eliminate pathogens [1, 2]. As a robust but non-targeted pathogen-killing defense mechanism, the microbicidal/cytotoxic components in NETs, like a double-edged sword, can also induce bystander “secondary” injury to vascular endothelia and adjacent cells. These injuries erode capillary-tissue barriers causing multi-organ dysfunction progressing to failure, even if the inciting infection is focal or decreasing [3, 4]. Cumulative research shows that increased NET-levels are associated with both severity of infection and risk for tissue injury, as seen in the association of increased NETs with COVID-19 severity [5, 6]and high mortality [7].
Preclinical studies show causal pathogenic mechanisms for NETs in SARS-CoV-2 virus infection, COVID-19. SARS-CoV-2 virus induces NET-formation in human healthy volunteer neutrophils [8], and the formed NETs cause injury in human epithelial and endothelial cells [7–9],including acute lung injury [10]. Concordantly, increased NET-levels have been implicated in all the clinical pathologies observed in the spectrum of severe COVID-19 multi-organ dysfunction including thromboses [11], parallel to observations in bacterial pneumonia and sepsis-induced models of acute lung injury or multi-organ failure (MOF) [12, 13]. The observed common pathogenic roles of excess NETs in secondary tissue injury, systemic micro-thrombosis or microvascular inflammation and occlusion, suggest reduction of NETs as a potential therapeutic approach to MOF, which to date remains without curative-intent therapy.
However, since NETs provide multiple key defense mechanisms against bacterial infections [2], sepsis [14] and viral infections [15], therapeutic approaches to block NETs-mediated secondary “bystander” tissue injury must target dysregulated NET-formation, but spare homeostatic regulated NET-formation. Given neutrophil heterogeneity and multiple mechanisms of NET-formation [16, 17], identification of dysregulated “rogue” NET-forming neutrophil subsets/subtypes that escape normal NET-clearance, accumulate, and contribute to multi-organ failure could be key to much-needed targeted therapies for severe COVID-19.
Our recent studies have identified DEspR + CD11b + neutrophils (DEspR + [Ns] from hereon), as a dysregulated “rogue” neutrophil-subset capable of NET-formation while in the circulation, extended survival, low-clearance, enhanced adhesion, and association with severity measures and mortality in COVID-19 acute respiratory distress syndrome (ARDS) [18–20]. Here, we test whether the putative “rogue” CD11b + DEspR + NET-forming neutrophil-subset, DEspR + [NET + N], mediates the worsening of early multi-organ dysfunction (measured by the Sequential Organ Failure Assessment [SOFA]-score [21] at timepoint-1) towards multi-organ failure (measured as higher SOFA-scores at a later timepoint-2), and/or mediates poor clinical outcomes (as measured by intensive care unit free days [ICUFD] by day-28) [22] in severe COVID-19 using causal mediation analysis. Identification of a rogue [NET + N] subset that mediates progression of multi-organ dysfunction in severe COVID-19 patient samples has the potential to identify a much-needed therapeutic target and/or biomarker. The combinatorial use of direct analysis of patient neutrophils and causal inference mediation statistics has the potential to validate a translational approach to overcoming low translatability of animal models in ARDS-multi-organ failure regardless of etiology.
Methods
Study design and participants
Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as most recently amended (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
A combined 2-site analysis of NET-forming neutrophil subsets from two independent prospective observational study cohorts previously characterized for ‘rogue’ neutrophils and NET-formation [18, 19]. At Boston Medical Center (BMC), the protocol study number is H-36744, study title: Humanized anti-DEspR antibody therapy for Acute Lung Injury (ALI/Acute Respiratory Distress Syndrome), and approval by Boston University’s Institutional Review Board on 12–01-2019. At Maine Medical Center (MMC), the protocol study number is 1598969–16, with study title “IT-19 Identification of molecular treatment targets for COVID-19”, and approval by Maine Medical Center’s Institutional Review Board on 5/8/2020. Clinical data and blood sample collections followed IRB approved protocols, (Supplemental Methods). Informed consent was obtained from the patient when able, or when unable, from the patient’s legally authorized representative (LAR). LAR-informed consents were obtained by phone using an IRB-approved informed consent-by-phone at BMC, or electronically at MMC, to minimize viral exposure. Clinical parameters of severity were obtained: non-neurologic SOFA score as a measure of multi-organ dysfunction and SaO2/FiO2 (SF)-ratio as a measure of respiratory distress/failure, and ICU-free days at day 28, with competing risk of death -1 (ICUFD) (22) as a summation outcome measure. Clinical measures were taken from two time points: timepoint-1 (t1) after informed consent after COVID19 diagnosis verification upon admission to the hospital or ICU, average 5.5 days, and timepoint-2 (t2): the day before ICU-death or ICU/hospital discharge. CBC-differential and blood samples for immunofluorescence cytology were obtained at timepoint-t1.
Rigor and reproducibility
Rigor was ascertained via compartmentalized blinding in research procedures. Clinical data collection was done blinded to determination and quantitation of [NET + N] subsets and vice versa. Quantitative imaging of [NET + N] subsets was done independently by a 3rd party blinded to clinical data. Causal mediation analysis was performed by researchers not involved in data collection (Supplemental Methods for detail).
Immunofluorescence cytology (IFC): immunofluorescence (IF)-staining of patient ‘blood smears’
Cytology slides were prepared directly from whole blood within 1 h from blood draw in EDTA-anticoagulated samples (BMC) [19, 23] and within 1-3 h from blood draw from acid-citrate dextrose samples (MMC) in order to preserve, hence detect circulating neutrophils with fragile DNA-webs/strands, as first observed in activated NET-forming neutrophils ex vivo by Brinkmann et al. 2004 [2]. To directly measure subset-specific [NET + Ns] in the circulation in a clinically feasible and safe way in COVID-19 patients, cytology slides were fixed in -20 °C 100% methanol prior to immunotyping for: 1] CD11b expression, an established marker of activated neutrophils in COVID-19 capable of NET-formation [2], and 2] DEspR expression expressed on rogue neutrophil-subset with extended lifespan and associated with severity in non-COVID-19 ARDS and COVID-19-ARDS [19], 3] 4′,6-diamidino-2-phenylindole (DAPI) staining for detection of DNA strands/webs still attached to CD11b + neutrophils, and 4] citrullinated Histone 3 as marker of decondensed histones characteristic of NET-formation [2, 24] (Supplemental methods for detail).
Semi-automated quantitation of [NET + Ns] subsets
Third party Nikon Imaging Laboratory (Cambridge MA) performed quantitative imaging analysis blinded to patient information for automated unbiased detection and quantitation of NET-forming DNA-extruding neutrophils by measuring the circularity index using a standard shape analysis formula 4pi (area/perimeter2) validated earlier [19]. Quantitative analysis of subsets of NET-forming neutrophils with molecular markers for CD11b ± and DEspR ± and DNA was performed via automated determination of fluorescence intensities of NET-forming neutrophils identified by circularity index < 0.8 (Supplemental Methods for detail).
Determination of measures of [NET + Ns]
From the IF-cytology analysis and quantitation of [NET + Ns], the % of DEspR + vs DEspR(-) [NET + Ns] was determined from the total NET + Ns detected with circularity index < 0.8 indicating extruded DNA. Data were exported to a CSV file, final scoring was completed in Excel. The number (#) of DEspR + vs DEspR(-) [NET + Ns] was calculated by multiplying the %DEspR + [NET + Ns] x ANC obtained on the same day complete blood count (CBC)-differential, #DEspR + [NET + Ns] or DEspR + [NET + N] counts.
Statistical analyses
Descriptive statistics and Spearman rank correlation analysis were performed using (PRISM 9.4, GraphPad). Power analyses were performed using SigmaStat 11.0.
Spearman correlation analysis
To select putative mediators for causal mediation analysis, we determined linear relationships of [NET + N] subset-specific levels with clinical severity measures relevant to the progression of multi-organ failure, we performed Spearman correlation analysis. For putative comparator mediators, we also analyzed DEspR + CD11b + neutrophil counts, neutrophil-to-lymphocyte ratio (NLR), and ANC. Spearman correlation coefficient (rS) greater than rS 0.46, for n = 36 subjects, was estimated to give power 0.8, at alpha 0.05. We performed Bonferroni correction of Spearman correlation P values: P x the number of hypotheses tested, in our study n = 7 hypotheses.
Causal Mediation analysis
(CMA) was performed using R (version 4.2.1) and package regmedint [25]. Causal mediation analysis seeks to disentangle relationships between three or more variables [26–28] where some or all of the effect of an exposure (A) on an outcome (Y) is mediated by a mediator of interest (M). Causal mediation analysis quantifies the direct effect of A on Y and the indirect effect of A on Y through the mediator M. In addition, causal mediation analysis accounts for interaction between the exposure and mediator such that the strength of the association of the indirect mediated pathway may depend on the value of the exposure and mediator variables.
Here, we examined DEspR + CD11b + NET + Ns as potential mediators of 1) progressive multi-organ dysfunction to multi-organ failure (from early t1-SOFA score to later t2-SOFA score), 2) progressive pulmonary specific organ dysfunction (early t1-SF to later t2-SF), and 3) t1-SOFA and t1-SF to length of hospital/ICU stay accounting for the competing risk of death (ICU free days [ICUFD]). We considered both mediator and interaction effects between t1 variables and DEspR + [NET + N] counts. For each causal pathway the primary estimand of interest was the percent mediated (i.e., the percent of the effect of the exposure on the outcome mediated by DEspR + [NET + N] counts) and the second estimand of interest was the percent eliminated (i.e., the percent of the effect of the exposure on the outcome that would be removed if DEspR + [NET + N] counts were reduced to zero). Because the exposures were continuous variables, we modeled the mediation effects of DEspR + [NET + N] counts due to a moderate change in the exposure variable (i.e., from its first quartile value to its third quartile value). The pure natural direct effect (the direct effect of exposure on outcome if the mediator [NET + Ns] is set at the value it would naturally take when exposure is at its reference value), the total natural direct effect (the direct effect of exposure on outcome accounting for exposure and mediator interaction), and the total effect (total effect of exposure on outcome through direct and indirect pathways) were also reported. As comparator, we also tested absolute neutrophil counts (ANC) as a mediator of t1-SOFA to t2-SOFA and t1-SOFA to ICUFD. We used bootstrapping with 10,000 replicates to calculate 95% confidence intervals.
Analysis of effects of a hypothetical treatment reducing DEspR + [NET + Ns] as mediator
We estimated the indirect effect on t2-SOFA, ICUFD, and t2-SF ratio mediated by DEspR + [NET + Ns] of a hypothetical treatment that would eliminate DEspR + [NET + Ns] as described by Lok and Bosch [29], which showed that a treatment effect on the mediator can be combined with off-treatment mediator and outcome data to estimate a causal indirect effect (but not a total or direct effect). To estimate the indirect effect on t2-SOFA and ICUFD mediated by DEspR + [NET + Ns] of a hypothetical treatment that would eliminate DEspR + [NET + Ns] (ie, zero-level), we included t1-SOFA ≥ 2 as a pre-treatment common cause of DEspR + [NET + Ns] and t2-SOFA/ICUFD, and also included t1-SOFA ≥ 4 as a second pre-treatment causal variable. For the t2-SF ratio, we included the t1-SF ratio as a pre-treatment common cause. For these analyses we modified existing SAS macros (29), for a continuous outcome modeled with linear regression similar to regmedint [25].
Results
Patient characteristics
COVID-19 subjects were enrolled from May to September 2020 prior to vaccinations or anti-viral therapies at Boston Medical Center and at Maine Medical Center. These subjects were analyzed independently earlier for “rogue” DEspR + CD11b + neutrophils using lab-specific flow cytometry experimental protocols respectively [18, 19]. Here, combined 2-site characteristics are summarized in Table 1. Of the 36 subjects, mean age 63.6 years, 67% were males, 67% met the Berlin Definition for ARDS (both male and female), 81% had corticosteroid therapy, 31% were diagnosed with clinical thrombosis, and 6% were placed on hemodialysis (Table 1). Among the 36 subjects, the median t1-SOFA-score was 2.5 (interquartile range [IQR] 1–6.25; range 0–12) and median t2-SOFA-score was 1 (IQR 0, 4.25; range 0–11). Median SF-ratios were 242.5 (IQR 135.8–346.2; range 77–457) at t1 and 334.5 (IQR 243.0–439.2; range 87.0–471.0) at t2. Now, we present the combined 2-site study of putative subsets of circulating NET-forming neutrophils analyzed in a common facility using identical protocols for immunotyping and semi-automated quantitation.
Table 1.
Summary of COVID-19 cohort characteristics
| Cohort Characteristics | N (%) |
|---|---|
| Male | 24 (66.7) |
| Race (White) | 18 (77.8) |
| BMI > 30 | 23 (63.9) |
| ARDS diagnosis | 24 (66.7) |
| Antibiotics | 26 (73.0) |
| Corticosteroid therapy | 29 (81.1) |
| Hemodialysis | 6 (16.7) |
| Thrombosis | 11 (30.8) |
| Deceased | 9 (25.0) |
| Median [IQR] | |
| Age (years) | 64.5 [56.5, 71.8] |
| # days from ICU admission t1 (n = 22) | 7 [3.0, 10.5] |
| # days from hospital admission t1 (n = 14) | 1 [0, 2] |
| ICUFD | 21.5 [-0.75, 28] |
| t1 SOFA-score | 2.5 [1.0, 6.8] |
| t2 SOFA-score | 1 [0, 4.8] |
| t1 SF-ratio | 242.5 [135.3, 350.8] |
| t2 SF-ratio | 334.5 [241.0, 441.8] |
| t1 NLR | 7.7 [4.9, 14.6] |
| t1 ANC | 4.3 [1.5, 8.9] |
| % t1 [DEspR + CD11b + NET + Ns] | 53.85 [28.2, 68.6] |
| % t1 [DEspR + CD11b + NET(-) Ns] | 46.15 [31.5, 71.8] |
| % t1 [DEspR(-)CD11b( ±) NET + Ns] | 0.94 [0.17, 3.00] |
| # t1 [DEspR + CD11b + NET + Ns] K/μL | 0.18 [0.02, 0.95] |
| # t1 [DEspR + CD11b + NET(-) Ns] K/μL | 0.15 [0.018, 1.1] |
| # t1 [DEspR(-)CD11b( ±) NET + Ns] K/μL | 0.03 [0.006, 0.080] |
| Ave [min–max] | |
| # t1 [DEspR + CD11b + NET + Ns] K/μL | 1.53 [0–15.4] |
| # t1 [DEspR + CD11b + NET(-) Ns] K/μL | 1.08 [0–7.9] |
| # t1 [DEspR(-)CD11b( ±) NET + Ns] K/μL | 0.11 [0–0.84 |
Circulating NET-forming neutrophil (NET + N) subsets in mod-severe acute COVID-19
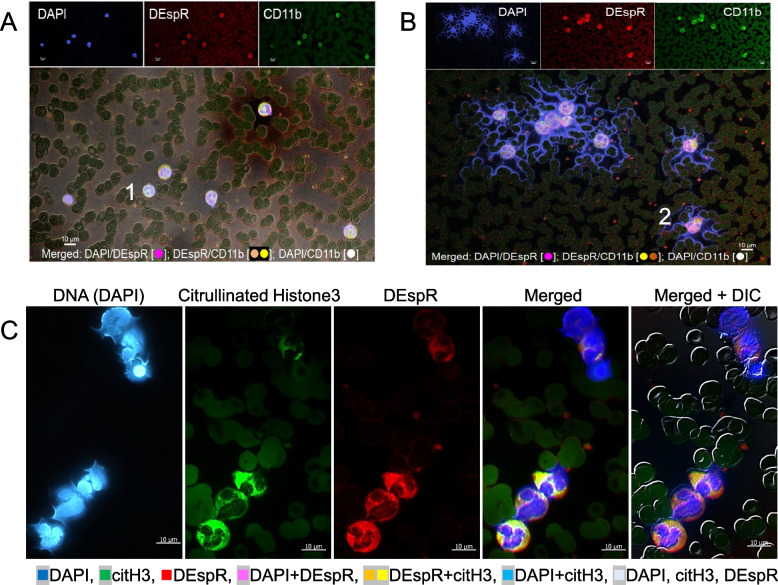
IFC-immunostaining of patient blood smears detected differential (CD11b ± DEspR ±) subset-specific levels of circulating NET + Ns in patients with severe COVID-19 (Table 1, Figs. 1 and 2). Representative IFC-images of [NET + Ns] show differences in a COVID-19 patient who survived (Fig. 1A) compared to a patient with multi-organ failure who died (Fig. 1B). IFC-immunostaining confirms NET-forming neutrophil features: [2, 24] extruded DNA, nuclear decondensation, histone-3 citrullination (citH3), plasma-membrane changes, neutrophil-derived microvesicles, (Fig. 1B-C).
Fig. 1.
Representative images of differential levels of circulating NET + Ns by immunofluorescence-cytology (IFC). A-B Representative IFC confocal microscopy image [z-stack through nucleus] showing minimal to no DEspR + CD11b + [NET + Ns] with extruded DNA (blue) in a (A) COVID-19 survivor, and (B) increased DEspR + [NET + Ns] in COVID-19 non-survivor. Bar 10 μm; DEspR + CD11b + microvesicles observed < 1 μm. Panel A #1 and Panel B #2: neutrophils shown in Fig. 2-A. C Representative IFC images of DEspR + [NET + Ns] showing from left to right: DAPI-DNA staining of decondensing nuclei in various stages of extrusion of DAPI-stained DNA, citrullinated histone-3 (citH3) expression with varying intensities, neutrophil DEspR + expression, merged image, and merged image with differential interference contrast (DIC) superimposed. Bar = 10 microns
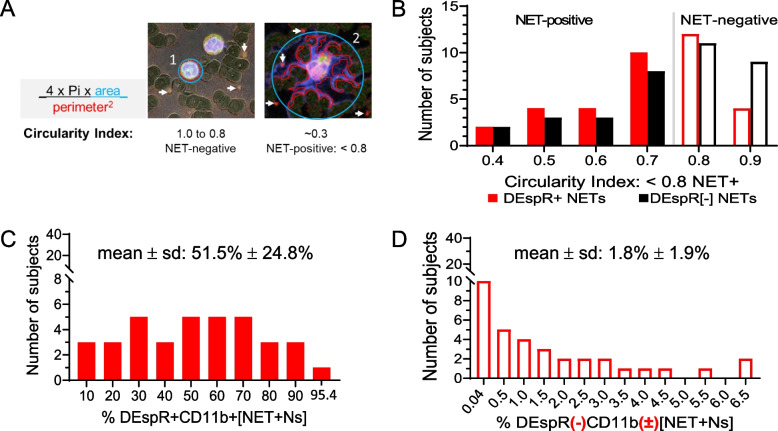
Fig. 2.
Comparative quantitative analysis of circulating NET + Ns using automated shape analysis in COVID-19 peripheral blood smears. A Diagram of circularity index determination and B corresponding frequency graph of the spread of circularity indices calculated by automated shape-analysis algorithm with designation of < 0.8 as cut-off for assignment of NET-forming neutrophils or [NET + Ns]. C Frequency curve of number of COVID-19 subjects (n = 36) with DEspR + CD11b + [NET + Ns]: median 53.4% (IQR: 28.2 – 68.5%), mean ± sd: 51.5% ± 24.8%. D Frequency curve of number of COVID-19 subjects (n = 36) with DEspR(-)CD11b( ±) [NET + Ns]: median 1.0% (IQR: 0.2 – 3.1%); mean ± sd: 1.8% ± 1.9%
Semi-automated quantitation of circulating [NET + N] subsets based on shape analysis of circularity (Fig. 2A), we detected both DEspR + and DEspR(-) [NET + N] subsets with differential frequencies (Fig. 2B) in our COVID-19 cohort. DEspR + [NET + Ns] comprised 51.5% ± 24.8% (mean ± SD) of circulating neutrophils (Fig. 2C), compared with 1.8% ± 1.9% (mean ± SD) DEspR(-) [NET + Ns] (Fig. 2D). Frequency distribution also showed a wide range of % DEspR + [NET + Ns] among all neutrophils in COVID-19 patients (Fig. 2C-D). Because of variations in absolute neutrophil counts, we derived the absolute number of DEspR + [NET + Ns] × 103 (K) per μL blood for subsequent analyses (Table 1).
Correlation of circulating DEspR + [NET + N] levels and severity measures
Spearman correlation analyses detected strong Spearman correlation coefficients (rS) ranging from 0.62–0.71 for DEspR + [NET + N] counts with clinical indicators of severity of multi-organ dysfunction at both time points (Table 2, Supplemental Fig. S1 for scatter plots). Notably, strong correlations were specific to DEspR + [NET + N] counts rather than % DEspR + [NET + Ns] with rS = 0.27–0.5. Concordantly, strong negative correlation of DEspR + [NET + N] counts and ICUFD was observed (rS = -0.63). Strong correlations of NLR and absolute neutrophil counts (ANC) with SOFA and SF-ratio at both timepoints were also observed (Table 2). Other subsets of NET-forming neutrophils: DEspR(-) [NET + Ns] (range rS 0.45 – 0.56), DEspR + [Ns] that are not NET-forming (range rS 0.44 – 0.60) exhibited weaker correlation coefficients (Table 2).
Table 2.
Spearman rank correlation coefficients (rs) of peripheral neutrophil-based markers with clinical measures of severity in acute COVID-19 patients (n = 36)
| Clinical Measures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Causal Variable-A t1-SOFA |
Outcome-A t2-SOFA |
Causal Variable-B t1-SF |
Outcome-B t2-SF |
Outcome-C ICUFD |
||||||
| Potential Mediator |
rs [95% C.I.] |
PB value |
rs [95% C.I.] |
PB value |
rs [95% C.I.] |
PB value |
rs [95% C.I.] |
PB value |
rs [95% C.I.] |
PB value |
| t1 #DEspR + [NET + Ns] |
0.71 [0.48, 0.84] |
1 × 10–5 |
0.62 [0.35, 0.79] |
7 × 10–4 |
-0.63 [-0.80, -0.38] |
2 × 10–4 |
-0.65 [-0.81, -0.40] |
1 × 10–4 |
-0.63 [-0.80, -0.37] |
3 × 10–4 |
| t1%DEspR + [NET + Ns] |
0.29 [-0.06,0.57] |
0.630 |
0.27 [-0.07,0.56] |
0.756 |
-0.50 [-0.72,-0.20] |
0.014 |
-0.29 [-0.57,0.05] |
0.602 |
-0.35 [-0.62,-0.02] |
0.245 |
| t1 #DEspR(-) [NET + Ns] |
0.51 [0.21, 0.72] |
0.007 |
0.47 [0.16, 0.70] |
0.027 |
-0.51 [-0.72, -0.20] |
0.014 |
-0.56 [-0.75, -0.27] |
0.003 |
-0.45 [-0.69, -0.14] |
0.042 |
| t1 #DEspR + [NET(-)Ns] |
0.60 [0.33,0.78] |
8 × 10–4 |
0.52 [0.22,0.73] |
0.009 |
-0.44 [-0.68,-0.12] |
0.049 |
-0.53 [-0.74,-0.23] |
0.006 |
-0.53 [-0.74,-0.24] |
0.007 |
| t1 #DEspR + total Ns |
0.67 [0.43,0.82] |
6 × 10–5 |
0.59 [0.31,0.77] |
0.001 |
-0.55 [-0.75,-0.26] |
0.007 |
-0.60 [-0.78,-0.33] |
7 × 10–4 |
-0.58 [-0.77,-0.31] |
0.001 |
| t1 NLR |
0.74 [0.53,0.86] |
2 × 10–6 |
0.51 [0.21,0.72] |
0.011 |
-0.51 [-0.72,-0.20] |
0.014 |
-0.50 [-0.72,-0.19] |
0.014 |
-0.57 [-0.76,-0.29] |
0.002 |
| t1 ANC |
0.71 [0.50,0.85] |
7 × 10–6 |
0.61 [0.35,0.79] |
7 × 10–4 |
-0.55 [-0.75,-0.26] |
0.007 |
-0.69 [-0.83,-0.46] |
2 × 10–5 |
-0.51 [-0.72,-0.21] |
0.014 |
Clinical Measures: ICUFD, ICU-free days by day 28 [28—ICUx days] with death scored as [-1] and > ICU-28 days scored as [0]; rs, Spearman correlation coefficient, rho; SF, SaO2/FiO2 ratio; SOFA-score, non-neurologic Sequential Organ Failure Assessment score; t1, earlier study timepoint on day of informed consent; t2, later study timepoint on day of discharge or death
Neutrophil-based Markers: #, 103 (K)/μL per microliter whole blood; ANC, absolute neutrophil count K/μL in whole blood; Ns, neutrophils; NET + Ns, NET-forming neutrophils; NLR, neutrophil to lymphocyte ratio; DEspR + [NET + Ns], DEspR + CD11b + NET-forming neutrophils; DEspR(-)[NET + Ns], DEspR-negative CD11b positive/negative [NET + Ns]; DEspR + [NET(-)Ns], DEspR + CD11b + neutrophils with circularity index > 0.8
Statistical analysis: Spearman Rank Order Correlation (GraphPad PRISM9.4). Correlation Coefficient rho (rs), rs > 0.46 for n = 36 attains power > 0.8, alpha 0.05. PB, p value with Bonferroni correction for the 7 hypotheses tested. 95% C.I., 95% Confidence Interval
In bold: max correlation coefficients in column
Variables for Causal Pathway Hypotheses: Exposure, the predictor or causal variable; Mediator, potential intermediate variable, and Outcome, the outcome variable
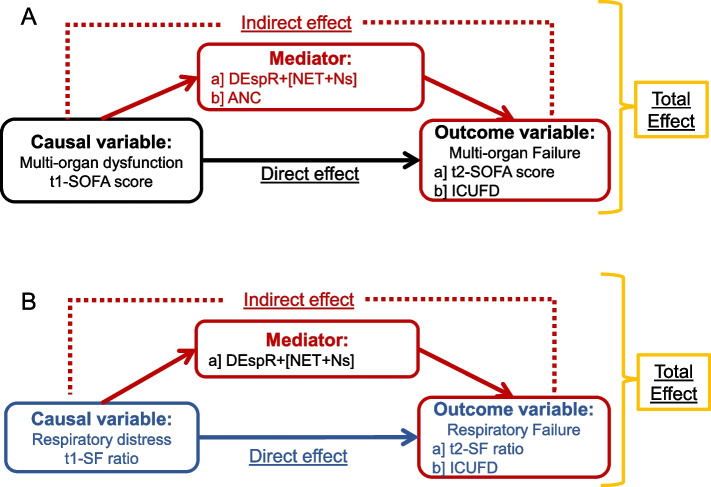
Causal mediation analysis of circulating DEspR + [NET + N] levels
Based on preclinical causal relationships of SARS CoV2, NET-formation, and injury to endothelial-tissue barrier in different vital organs contributing to multi-organ failure and supported by corresponding correlation analyses depicting linear relationships (Table 2, Supplemental Fig. S1), we tested causal path hypotheses for progression of multi-organ failure (Fig. 3-A) and progression of respiratory failure (Fig. 3-B). As shown in Table 3, Mediation Analysis estimated that 44.1% [95% CI: 16.5, 110.6] of the relationship between t1-SOFA (modeling a change in t1-SOFA from its first quartile value [1 point] to its third quartile value [6.25 points]) and t2-SOFA (outcome) was mediated by DEspR + [NET + Ns] and that 46.9% [95% CI: 15.8, 124.6] of the effect of t1-SOFA on t2-SOFA would be eliminated by reducing DEspR + [NET + Ns] to 0. Similarly, in the proposed causal pathway between t-1 SOFA and ICUFD, 47.1% [22.0, 72.3%] of the relationship was mediated by DEspR + [NET + Ns] and 51.1% [95% CI: 22.8, 80.4] of the relationship between t1-SOFA and ICUFD would be eliminated by reducing DEspR + [NET + Ns] to 0. Concordantly, analysis of a hypothetical therapeutic that would reduce DEspR + [NET + Ns] to zero in patients with t1-SOFA ≥ 2 predicted a decrease in t2-SOFA of 0.98 [95% CI: 0.29, 2.06] points as compared with no therapeutic, and for patients with t1-SOFA ≥ 4, a decrease in t2-SOFA of 1.4 [95% CI: 0.47, 3.05] points (Table 3), indicative of a decrease in progression of multi-organ failure.
Fig. 3.
Directed acylic graphs of causal hypotheses for mediation analysis of hypothesized mediators: DEspR + [NET + Ns] or comparator ANC in COVID-19. A Hypothesis-1: DEspR + [NET + Ns] mediate progression of multi-organ dysfunction causal path between t1 (t1-SOFA score) to multi-organ failure (t-2 SOFA score) or poor clinical outcomes (ICUFD). Alternative mediator tested: ANC, absolute neutrophil counts. B Hypothesis-2: DEspR + [NET + Ns] mediate progression of respiratory distress at t1 (t1-SF ratio) to respiratory failure at t2 (t2-SF) or poor clinical outcomes (ICUFD). SOFA-score, obtained without neurological component; ICUFD, ICU-free days by day 28 with 0 for patients in the ICU > 28 days and competing risk of death [-1]
Table 3.
Causal Mediation Analysis of [NET+Ns] as mediator of multi-organ failure in severe COVID-19
| Exposure (causal variable): t1-SOFA, or t1-SF Mediator: [NET+Ns], or [ANC] Outcome: t2-SOFA, or ICUFD, or t2-SF-ratio |
Natural direct effecta | Natural indirect effectb | Total Effectc | Percent mediatedd | Percent eliminatede | Indirect effect of eliminating [NET+Ns]f-h |
|---|---|---|---|---|---|---|
| Causal pathway hypotheses |
Effect (95% CI) |
Effect (95% CI) |
Effect (95% CI) |
Effect (95% CI) |
Effect (95% CI) |
Effect (95% CI) |
|
A] Direct effect: t1-SOFA→ t2-SOFA Indirect effect: t1-SOFA→[NET+Ns]→t2-SOFA |
3.3 (2.0, 4.7) |
2.6 (-1.4, 5.9) |
5.9 (1.7, 9.9) |
44.1 (16.5, 110.6) |
46.9 (15.8, 124.6) |
0.98 f (0.29, 2.06) |
|
B] Direct effect: t1-SOFA → ICUFD Indirect effect: t1-SOFA →[NET+Ns]→ICUFD |
-12.3 (-17.9, -6.2) |
-11.0 (-18.5, 4.3) |
-23.3 (-33.0, -6.3) |
47.1 (22.0, 72.3) |
51.1 (22.8, 80.4) |
3.0 g (0.85, 7.09) |
|
C] Direct effect: t1-SF → t2-SF Indirect effect: t1-SF →[NET+Ns]→t2-SF |
124 (-263, 364) |
46 (-224, 481) |
170 (103, 235) |
27.2 (-107, 372) |
15.6 (-9.7, 64.2) |
16.8 h (-4.6, 42.8) |
|
D] Direct effect: t1-SF → ICUFD Indirect effect: t1-SF →[NET+Ns]→ICUFD |
1.7 (-18.1, 43.9) |
13.8 (-36.0, 34.3) |
15.4 (5.1, 20.7) |
89.3 (-131, 225.5) |
22.6 (-15.5, 43.4) |
|
|
E] Direct effect: t1-SOFA → t2-SOFA Indirect effect: t1-SOFA →[ANC]→t2-SOFA |
2.5 (1.0, 4.3) |
1.1 (-0.7, 2.2) |
3.6 (1.9, 4.8) |
31.1 (-18.0, 63.0) |
36.5 (-28.2, 70.8) |
|
|
F] Direct effect: t1-SOFA → ICUFD Indirect effect: t1-SOFA →[ANC]→ICUFD |
-13.6 (-22.0, -6.8) |
-1.1 (-8.7, 6.2) |
-14.6 (-21.0, -9.5) |
7.4 (-45.5, 77.8) |
0.1 (-54.7, 67.0) |
Legend: Causal effect estimates are for t1-SOFA from Q1-Q3 (1 to 6.25), and for t1-SF ratio from Q1-Q3 (135.8 to 346.2) in this prospective pilot cohort. [ANC], absolute neutrophil counts from CBC-differential; ICUFD, ICU-free days at day 28 with death as [-1]; NET, neutrophil extracellular traps; [NET+Ns], immunotyped (DEspR+CD11b+)NET-forming neutrophil counts (103[K]/μL); SF, SF ratio SpO2/FiO2; SOFA, sequential organ failure assessment score (points) without neurological component; t1, time of consented blood sample draw for analysis of NET+N levels; t2, time before ICU/hospital-discharge or ICU-death < 28 days; 95% CI (n, m), were calculated using bootstrapping with 10,000 replicates
aNatural direct effect: the direct effect of exposure on outcome if the mediator [NET+Ns] is set at the value it would naturally take when SOFA-1 score is at its reference value
bNatural indirect effect: the effect of exposure on outcome mediated by DEspR+[NET+Ns], accounting for exposure and mediator interaction
cTotal Effect: the total effect of exposure on outcome through direct and indirect pathways
dThe percent of the effect of the exposure on the outcome mediated by DEspR+[NET+N] counts
eThe percent of the effect of exposure on the outcome that would be eliminated by reducing the DEspR+[NET+N] count to 0 (total effect - controlled direct effect under a hypothetical situation where DEspR+[NET+N] is 0) / total effect)
fIn patients with t1-SOFA ≥ 2, the indirect effect of eliminating [NET+Ns] decreased t2-SOFA by 0.98 points (95% CI: 0.27,2.13), ~15% absolute risk reduction (arr) (37). In patients with t1-SOFA ≥ 4, the indirect effect of eliminating [NET+Ns] is 1.41 95% CI (0.47,3.05), ~22% arr
gIn patients with t1-SOFA ≥ 2, outcome model fit in those with t1-SOFA ≥ 2; 50% rrr, relative risk reduction compared to corticosteroid therapy (38-39)
hIn patients with t1-SF ratio <300, outcome model fit in those with t1-SF ratio <300, not including interaction (p>0.77)
In contrast, mediation analysis for progression to acute respiratory failure (Fig. 3-B) showed no significant mediation by DEspR + [NET + Ns] between t1-SF and t2-SF, and between t1-SF and ICUFD (Table 3). Additionally, despite strong correlations with t2-SOFA and ICUFD (Table 2), there was no significant evidence of mediation by ANC on both causal path hypotheses for progression of multi-organ failure (Fig. 3-A): from t1-SOFA to t2-SOFA and from t1-SOFA to ICUFD (Table 3).
Discussion
Premised upon preclinical studies detecting a causal role of SARS CoV2 on NET-formation [8] and NET-forming neutrophils on capillary-tissue barriers, [8, 9]this prospective study of patients with acute COVID-19 identified DEspR + [NET + Ns] as a mediator of multi-organ failure progression. These observations are supported by the causal role of NETs in direct endothelial cell injury demonstrated in ex vivo studies [8–10, 30], and the causal role of DEspR in extending neutrophil lifespan [20]. We note that while causal mediation analysis has been previously used to evaluate inflammatory mediators of the effect of obesity on risk for mortality in COVID-19 [31], and soluble RAGE receptor levels and angiopoietin-2-levels in sepsis-related ARDS [32, 33], here we report causal mediation analysis of NET-forming neutrophil subsets.
The differential mediation effect profiles for DEspR + [NET + Ns] compared with ANC, despite similarly strong correlations with multi-organ failure outcomes, highlight the emerging role of NET + Ns as a central mechanism for neutrophil-mediated secondary tissue injury and/or immuno-thrombosis in multi-organ failure in COVID-19 [7, 34–36], and the importance of neutrophil subset specific analysis as ANC represents the total mixture of neutrophil-subsets. Likewise, the differential mediation of worse SOFA score and ICUFD – but not of worse SF-ratio – by DEspR + [NET + Ns] – indicates specificity of causal effect estimates of mediation. This differential mediation supports the hypothesis that the low SF-ratio in COVID-19 is caused by the direct damage of respiratory epithelia infected with the SARS CoV2 virus [37], rather than by neutrophil-mediated tissue injury of indirect ARDS [38]. Taken together, concordance of our findings supports the pathogenic role of circulating [NET + Ns] in direct endothelial injury and microcirculation compromise in the progression of secondary multi-organ failure in COVID-19.
In the analysis of a hypothetical treatment that eliminates DEspR + [NET + Ns] among patients with a SOFA score of 2 or more, elimination of DEspR + [NET + Ns] was associated with an indirect effect of a 1-point decrease in subsequent SOFA score, which for patients with COVID-19 ARDS, would translate approximately to a 15% absolute risk reduction (arr) in death [39]. Similarly, elimination of DEspR + [NET + Ns] was associated with a 3-day increase in ICUFD. These results suggest that a novel treatment that eliminates DEspR + [NET + Ns] could potentially reduce mortality in severe COVID-19 with an effect estimate at least as strong as that of corticosteroids [40, 41]. Because corticosteroids, especially dexamethasone, induce neutrophilia [42, 43], increase neutrophil lifespan [44–46], and have no effect on NETs [47], the potential contribution of the hypothetical elimination of DEspR + [NET + Ns] leading to a reduction in mediation effect, could be additive or synergistic with reported dexamethasone efficacy in reducing severe acute COVID-19 mortality.
Notably, preclinical studies support the feasibility of this therapeutic hypothesis as the anti-DEspR antibody induces apoptosis in DEspR + neutrophils observed on live cell imaging of macaque neutrophils and promotes neutrophil apoptosis without worsening elevated myeloperoxidase and complement activation levels, as observed in an ex vivo experimental system testing ARDS patient whole blood samples [19]. Intuitively, the induction of apoptosis in circulating “rogue” [DEspR + CD11b +] neutrophils will pre-empt DEspR + [NET + Ns], but spare DEspR(-)[NET + Ns] and DEspR(-)neutrophil roles in key defense mechanisms against pathogens, thus likely avoiding the increases in infection risk seen in total neutrophil/NET + N inhibition or depletion.
Concordantly, data also reveal new concepts. The strong correlation of absolute number of DEspR + [NET + Ns] (range rS 0.62 – 0.71) compared with an alternative parameter, the % of DEspR + [NET + Ns] (range rS 0.27 – 0.5), suggests that the % DEspR + [NET + N] parameter is insufficient as absolute neutrophils counts vary among COVID-19 patients, hence making it possible that a high % but low ANC could be numerically and pathogenically equivalent to a low % but high ANC level. This suggests a circulating "[NET + N]-burden” hypothesis in progression of multi-organ failure, parallel to tumor cell-burden in cancer progression [48]. The strong correlation of DEspR + [NET + Ns] with SOFA-score we report here as compared with that previously observed for plasma NET-biomarker MPO-DNA complexes [7] indicates the advantage of quantitative IF-cytology (qIFC) subset-specific analysis of circulating DEspR + [NET + Ns], and that qIFC of whole blood smears provides a pathogenically relevant measure of circulating subset-specific NET-forming neutrophil levels. This method opens the door to identification and comparative analysis of other molecular subsets of NET-forming neutrophils in COVID-19 as potential causal intermediate(s) in different critical care pathologies wherein NETs are implicated. Lastly, if borne out in development of novel therapeutics, causal mediation analyses may be a useful tool to translate preclinical causality and efficacy to validated therapeutic hypotheses for clinical trial and address the unmet need arising from cumulative low translatability of preclinical animal models of ALI/ARDS to clinical trial efficacy. This potential is supported by an earlier study showing that causal mediation analysis can identify the promising treatment among different candidates for further testing in randomized clinical trials [29].
Limitations of study
Our small cohort size may be underpowered to detect weaker associations. This observational study was done during the first phase of COVID-19 without vaccination or anti-viral therapies available; thus, how these therapies may modify the relationships between organ dysfunction and NETs is unclear. Since NET levels vary with time and t1 was not the same for all patients due to informed consent issues, the study design may have introduced t1 noise/variation that decreases power. Additionally, although measurement of the indirect effect was imprecise (i.e., the 95% CIs crossed 0), DEspR + [NET + Ns] is estimated to mediate a substantial proportion of the relationship between t1 SOFA and t2 SOFA. Translational research in critically ill patients is limited as to experimental sampling frequency and amount, but nevertheless provides invaluable pathophysiological context in hypothesis validation for further study.
Conclusions and clinical implications
In this prospective pilot study, Causal Mediation Analysis detected DEspR + [NET + N] subset as a mediator of progression of multi-organ-dysfunction in COVID-19 and the hypothetical reduction of DEspR + [NET + N] subset support the therapeutic hypothesis that prevention or reduction of DEspR + [NET + Ns] has the potential to reduce progression to multi-organ failure in severe acute COVID-19. Altogether, data provide translational milestones in support of further studies to advance DEspR + [NET + Ns] as a much-needed potential biomarker for patient stratification and therapeutic target for multi-organ failure in severe acute COVID-19.
Supplementary Information
Additional file 1: Supplemental Methods. Figure S1. Scatter plots and Spearman correlation coefficients of DEspR+[NET+N] counts (103or K/μL)with measures of severity in COVID-19 at timepoint-1 (t1) and timepoint-2 (t2).
Acknowledgements
Not applicable.
Authors’ contributions
NAB, NR-O, DBS, SVR, AJW, VLMH contributed to the concepts and design of the study; NAB, AJW, JJL causal mediation analysis and review; NRO data collation for causal mediation and correlation analyses; DBS and BUSM General Clinical Research Unit core recruited patients; DBS, JTdK, MQN collected clinical data and patient samples; AJW, DBS corroborated ARDS diagnosis at BMC and MMC respectively; MQN, KAL IF-cytology staining; VLMH IF-cytology quality-control review prior 3rd party quantitative analysis; GCRU-core, JTdK prepared cytology slides; drafting of manuscript, VLMH, NRO, NAB, DBS, SVR, JJL, AJW; all authors: review of manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by: Boston Biomedical Innovation Center Drive Grants (NIH NHLBI 5U54HL 119145–07), match funds from Boston University Chobanian and Avedisian School of Medicine and Department of Medicine to NRO and VLMH; support from Clinical Translational Science Institute’s General Clinical Research Center (NIH 1UL1TR001430), NIH/NCATS 1KL2TR001411 to NAB. Work done at Maine Medical Center was supported by a Northern New England Center for Clinical and Translational Research seed grant under (NIH/NIGMS 5U54GM11516-03), and by NControl Therapeutics gift. Nikon Imaging Lab live-cell imaging and quantitative analysis was supported by NControl Therapeutics. All funding agencies did not participate in the design, conception, data acquisition, analysis or interpretation of data, or manuscript preparation.
Availability of data and materials
Data are available from corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as most recently amended (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
At Boston Medical Center (BMC), the protocol study number is H-36744, study title: Humanized anti-DEspR antibody therapy for Acute Lung Injury (ALI/Acute Respiratory Distress Syndrome), and approval by Boston University’s Institutional Review Board on 12–01-2019. At Maine Medical Center (MMC), the protocol study number is 1598969–16, with study title “IT-19 Identification of molecular treatment targets for COVID-19”, and approval by Maine Medical Center’s Institutional Review Board on 5/8/2020. Clinical data and blood sample collections followed IRB approved protocols.
Consent for publication
Not applicable.
Competing interests
Boston University holds awarded patents on DEspR as novel therapeutic approach for cancer, stroke, COVID-19, NETosis and activated neutrophils. VLMH and NRO are co-inventors in patents filed by Boston University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Victoria L. M. Herrera, Nicholas A. Bosch and Judith J. Lok are co-first authors.
David B. Seder, Nelson Ruiz-Opazo and Allan J. Walkey are co-senior authors.
Contributor Information
Victoria L. M. Herrera, Email: vherrera@bu.edu
Nicholas A. Bosch, Email: nabosch@bu.edu
Judith J. Lok, Email: jjlok@math.bu.edu
Mai Q. Nguyen, Email: mainguyen27197@gmail.com
Kaitriona A. Lenae, Email: klenae@bu.edu
Joanne T. deKay, Email: Joanne.deKay@mainehealth.org
Sergey V. Ryzhov, Email: Sergey.Ryzhov@mainehealth.org
David B. Seder, Email: David.Seder@mainhealth.org
Nelson Ruiz-Opazo, Email: nruizo@bu.edu.
Allan J. Walkey, Email: alwalkey@bu.edu
References
- 1.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Scozzi D, Liao F, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophil extracellular traps in acute lung injury. Front Immunol. 2022;13:953195. doi: 10.3389/fimmu.2022.953195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huckriede J, Anderberg SB, Morales A, de Vries F, Hultström M, Bergqvist A, et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci Rep. 2021;11:15701. doi: 10.1038/s41598-021-95209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva CMS, Wanderley CWS, Veras FP, Sonego F, Nascimento DC, Gonçalves AV, et al. Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology. Crit Care. 2022;26:206. doi: 10.1186/s13054-022-04062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv D, Xu Y, Cheng H, Ke Y, Zhang X, Ying K. A novel cell-based assay for dynamically detecting neutrophil extracellular traps-induced lung epithelial injuries. Exp Cell Res. 2020;394:112101. doi: 10.1016/j.yexcr.2020.112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thierry AR, Roch B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J Clin Med. 2020;9:2942. doi: 10.3390/jcm9092942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3:e98178. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Jenne CN, Kubes P. Virus-induced NETs–critical component of host defense or pathogenic mediator? PLoS Pathog. 2015;11:e1004546. doi: 10.1371/journal.ppat.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 18.deKay JT, Emery IF, Rud J, Eldridge A, Lord C, Gagnon DJ, et al. DEspRhigh neutrophils are associated with critical illness in COVID-19. Sci Rep. 2021;11:22463. doi: 10.1038/s41598-021-01943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera VLM, Walkey AJ, Nguyen MQ, Gromisch CM, Mosaddhegi JZ, Gromisch MS, et al. A targetable 'rogue' neutrophil-subset, [CD11b+DEspR+] immunotype, is associated with severity and mortality in acute respiratory distress syndrome (ARDS) and COVID-19-ARDS. Sci Rep. 2022;12:5583. doi: 10.1038/s41598-022-09343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carstensen C, Müller M, Tan GLA, Pasion KA, Hohlfeld JM, Herrera VLM, et al. “Rogue” neutrophil-subset [DEspR+CD11b+/CD66b+] immunotype is an actionable therapeutic target for neutrophilic inflammation-mediated tissue injury – studies in human, macaque and rat LPS-inflammation models. Front Immunol. 2022;13:1008390. doi: 10.3389/fimmu.2022.1008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Auriemma CL, Taylor SP, Harhay MO, Courtright KR, Halpern SD. Hospital-Free Days: A Pragmatic and Patient-centered Outcome for Trials among Critically and Seriously Ill Patients. Am J Respir Crit Care Med. 2021;204:902–909. doi: 10.1164/rccm.202104-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera VLM, Takahashi CE, Nguyen MQ, Mosaddeghi JZ, Denis R, Greer DM, et al. "Rogue" [DEspR+CD11b+] neutrophil subset correlates with severity in spontaneous intracerebral hemorrhage. Front Neurol. 2022;13:935579. doi: 10.3389/fneur.2022.935579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirose T, Hamaguchi S, Matsumoto N, Irisawa T, Seki M, Tasaki O, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS ONE. 2014;9:e111755. doi: 10.1371/journal.pone.0111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida K, Li Y: regmedint: Regression-Based Causal Mediation Analysis with Interaction and Effect Modification Terms. R package version 1.0.0. Available at: https://kaz-yos.github.io/regmedint/. Accessed 2022
- 26.Zhang Z, Zheng C, Kim C, Van Poucke S, Lin S, Lan P. Causal mediation analysis in the context of clinical research. Ann Transl Med. 2016;4:425. doi: 10.21037/atm.2016.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ. Mediation Analysis: A Practitioner's Guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 29.Lok JJ, Bosch RJ. Causal Organic Indirect and Direct Effects: Closer to the Original Approach to Mediation Analysis, with a Product Method for Binary Mediators. Epidemiology. 2021;32:412–420. doi: 10.1097/EDE.0000000000001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Block H, Rossaint J, Zarbock A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells. 2022;11:1919. doi: 10.3390/cells11121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foulkes AS, Selvaggi C, Shinnick D, Lumish H, Cao T, Thaweethai T, et al. Understanding the Link Between Obesity and Severe COVID-19 Outcomes: Causal Mediation by Systemic Inflammatory Response. J Clin Endocrinol Metab. 2022;107:e698–e707. doi: 10.1210/clinem/dgab629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones TK, Feng R, Kerchberger VE, Reilly JP, Anderson BJ, Shashaty MGS, et al. Plasma sRAGE Acts as a Genetically Regulated Causal Intermediate in Sepsis-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201:47–56. doi: 10.1164/rccm.201810-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilly JP, Wang F, Jones TK, Palakshappa JA, Anderson BJ, Shashaty MGS, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44:1849–1858. doi: 10.1007/s00134-018-5328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackermann M, Anders HJ, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;28:3125–3139. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Chen X, Liu X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front Immunol. 2022;13:838011. doi: 10.3389/fimmu.2022.838011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deinhardt-Emmer S, Böttcher S, Häring C, Giebeler L, Henke A, Zell R, et al. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J Virol. 2021;95:e00110–e121. doi: 10.1128/JVI.00110-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vassallo A, Wood AJ, Subburayalu J, Summers C, Chilvers ER. The counter-intuitive role of the neutrophil in the acute respiratory distress syndrome. Br Med Bull. 2019;131:43–55. doi: 10.1093/bmb/ldz024. [DOI] [PubMed] [Google Scholar]
- 39.Fayed M, Patel N, Angappan S, Nowak K, Vasconcelos Torres F, Penning DH, et al. Sequential Organ Failure Assessment (SOFA) Score and Mortality Prediction in Patients With Severe Respiratory Distress Secondary to COVID-19. Cureus. 2022;14:e26911. doi: 10.7759/cureus.26911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 41.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 42.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 43.Mishler JM, Emerson PM. Development of Neutrophilia by serially increasing doses of dexamethasone. Br J Haematol. 1977;36:249–257. doi: 10.1111/j.1365-2141.1977.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 44.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–3188. doi: 10.1182/blood.V86.8.3181.3181. [DOI] [PubMed] [Google Scholar]
- 45.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–25. doi: 10.4049/jimmunol.154.9.4719. [DOI] [PubMed] [Google Scholar]
- 46.Kato T, Takeda Y, Nakada T, Sendo F. Inhibition by dexamethasone of human neutrophil apoptosis in vitro. Nat Immun. 1995;14:198–208. [PubMed] [Google Scholar]
- 47.Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345:430–437. doi: 10.1124/jpet.112.202879. [DOI] [PubMed] [Google Scholar]
- 48.Sakata Y, Kawamura K, Ichikado K, Shingu N, Yasuda Y, Eguchi Y, et al. Comparisons between tumor burden and other prognostic factors that influence survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2019;10:2259–2266. doi: 10.1111/1759-7714.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Methods. Figure S1. Scatter plots and Spearman correlation coefficients of DEspR+[NET+N] counts (103or K/μL)with measures of severity in COVID-19 at timepoint-1 (t1) and timepoint-2 (t2).
Data Availability Statement
Data are available from corresponding author upon reasonable request.