Abstract
Objective
To analyse the clinical profile of SARS-CoV-2 breakthrough infections in at least double-vaccinated patients with inflammatory rheumatic diseases (IRDs).
Methods
Data from the physician-reported German COVID-19-IRD registry collected between February 2021 and July 2022 were analysed. SARS-CoV-2 cases were stratified according to patients’ vaccination status as being not vaccinated, double-vaccinated or triple-vaccinated prior to SARS-CoV-2 infection and descriptively compared. Independent associations between demographic and disease features and outcome of breakthrough infections were estimated by multivariable logistic regression.
Results
In total, 2314 cases were included in the analysis (unvaccinated n=923, double-vaccinated n=551, triple-vaccinated n=803, quadruple-vaccinated n=37). SARS-CoV-2 infections occurred after a median of 151 (range 14–347) days in patients being double-vaccinated, and after 88 (range 14–270) days in those with a third vaccination. Hospitalisation was required in 15% of unvaccinated, 8% of double-vaccinated and 3% of triple-vaccinated/quadruple-vaccinated patients (p<0.001). Mortality was 2% in unvaccinated, 1.8% in the double-vaccinated and 0.6% in triple-vaccinated patients. Compared with unvaccinated patients, double-vaccinated (OR 0.43, 95% CI 0.29 to 0.62) and triple-vaccinated (OR 0.13, 95% CI 0.08 to 0.21) patients showed a significant lower risk of COVID-19-related hospitalisation. Using multivariable analysis, the third vaccination was significantly associated with a lower risk for COVID-19-related death (OR 0.26; 95% CI 0.01 to 0.73).
Conclusions
Our cross-sectional data of COVID-19 infections in patients with IRD showed a significant reduction of hospitalisation due to infection in double-vaccinated or triple-vaccinated patients compared with those without vaccination and even a significant reduction of COVID-19-related deaths in triple-vaccinated patients. These data strongly support the beneficial effect of COVID-19 vaccination in patients with IRD.
Trial registration number
EuDRACT 2020-001958-21.
Keywords: COVID-19, vaccination, antirheumatic agents, inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
In patients with inflammatory rheumatic disease (IRD), the use of SARS-CoV-2 vaccines is well tolerated and not associated with a significant increase of disease activity.
Recent data indicate the necessity of a third dose of SARS-CoV-2 vaccination to decrease the risk of severe SARS-CoV-2, especially in vulnerable patients.
WHAT THIS STUDY ADDS
In this group of patients with IRD, double-vaccinated and triple-vaccinated patients showed a significant lower rate of COVID-19-related hospitalisation.
Triple-vaccinated patients had a significant reduction of COVID-19-related deaths.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results support the recommendation to administer a third dose of COVID-19 vaccine in patients with IRD.
In vulnerable double-vaccinated patients with IRD, the use of monoclonal antibodies or antiviral drugs might be considered to reduce the risk of COVID-19-related death.
Introduction
Recent data show that individuals who have had a booster (triple) vaccination appear to be better protected against SARS-CoV-2—especially against the Omicron variant—than those who received double vaccination only.1 In addition, the safety profiles of SARS-CoV-2 vaccines have been shown to be comparable in patients with inflammatory rheumatic diseases (IRDs) and individuals not suffering from IRD.2
The majority of patients with IRD develop a sufficient immune response after two-dose SARS-CoV-2 messenger RNA (mRNA) vaccination, although lower median antibody titres were observed in patients treated with mycophenolate and rituximab.3 Cytokine inhibitors (eg, tumour necrosis factor inhibitors, TNFi), by contrast, do not seem to hamper sufficient SARS-CoV-2 antibody responses after vaccination.3 4
In summer 2021, several countries started to administer booster vaccine doses, primarily to elderly and vulnerable individuals, including immunocompromised individuals and healthcare workers.5 In a small cohort of patients with IRD, the third (‘booster’) vaccination was followed by a substantial increase of antibody titres against SARS-CoV-2 spike protein in 80% of the patients who did not develop sufficient or even any titres on their initial vaccine series.6 However, infection with SARS-CoV-2 has been observed in individuals after full vaccination, including patients with IRD.7 8 In a case report, two patients with IRD with inflammatory joint diseases under treatment with TNFi were described who contracted SARS-CoV-2 infection after three doses of mRNA SARS-CoV-2 vaccination with a mild course and without the need for hospitalisation.7 In 197 partially or double-vaccinated (DV) patients with IRD, breakthrough infections were reported.8 More than half of the DV individuals who required hospitalisation received B-cell depleting treatment for their IRD before infection.8 This study did not include data on breakthrough infection after a third dose of vaccination.8
So far, little is known about the frequency and the course of SARS-CoV-2 ‘breakthrough’ infections in patients with IRD, who have been vaccinated twice or three times.
To investigate the frequency and course of SARS-CoV-2 breakthrough infections among patients with IRD, who had been vaccinated at least two times, data from the German COVID-19-IRD registry were analysed.
Methods
Data from February 2021 to July 2022 were included in our analysis. Patients with double vaccination (primary course of vaccination) against COVID-19, at least 14 days prior to documented SARS-CoV-2 infection, were identified. Only cases of first SARS-CoV-2 infections were considered, no cases of reinfections. Beyond the type of IRD, immunomodulation as well as comorbidities, dates and type of vaccinations, and specifically the outcome of the SARS-CoV-2 infection were compared with unvaccinated (UV) patients with IRD who had contracted SARS-CoV-2. COVID-19-related complications, such as concomitant infections, thromboembolic events, myocarditis, heart failure and new onset of arrhythmia, were also recorded.
Data source
The German COVID-19 registry for patients with IRD was launched in March 2020 by the German Society for Rheumatology together with the Justus-Liebig-University Giessen. Rheumatologists from all over Germany voluntarily entered data on patients with IRD with SARS-CoV-2 infection into a web-based registry with implemented plausibility checks (www.covid19-Rheuma.de; for more details, see reference 9). Patients with a known IRD and positive testing for SARS-CoV-2 were included by their treating rheumatologists after registration. Patients had to have positive PCR-swabs for SARS-CoV-2 for inclusion in our registry. In contrast to other registries, presumptive diagnosis of COVID-19 based on suggestive symptoms, X-ray or CT findings or on other ‘unknown’ findings was not sufficient to enter the patients into the database. The definition of ‘absence of immunomodulation’ excludes all treatments with DMARDs and/or glucocorticoids. The definition of ‘absence of relevant comorbidities’ excludes comorbidities such as cardiovascular diseases (eg, heart failure, myocardial infarction, coronary artery disease), arrhythmia, arterial hypertension, bronchial asthma, chronic obstructive pulmonary disease, interstitial lung disease, chronic renal failure, liver cirrhosis, osteoporosis, pregnancy, diabetes mellitus, pulmonary hypertension, cancer/history of cancer, other relevant physician-reported comorbidities (individual input).
At the end of December 2020, vaccine-related questions were added to the registry, including whether patients had received a COVID-19 vaccine, and if so, which vaccine was given, how many doses and the dates of all received COVID-19 vaccinations.
Statistical analysis
Patients’ characteristics were first analysed descriptively for each group (UV, DV, triple-vaccinated (TV) and quadruple-vaccinated (QV)). Due to the limited case number of QV breakthrough infections (n=37), further investigations focused on UV, DV and TV cases. Median was calculated for age, body mass index and time elapsed between second/third vaccine dose and SARS-CoV-2 infection. Categorical variables are reported as numbers and percentages. Groups were compared using non-parametric (χ2, Fisher’s exact test, Kruskal-Wallis’ test) tests, depending on the measurement level of the variables and cell sizes in crosstabs. P values were calculated.
Univariate logistic regression was used to assess the individual association between COVID-19-related hospitalisation or COVID-19-related death (dependent variables) and patients’ vaccination status. Age, gender, use of TNFi, the absence of immunomodulation and the absence of relevant comorbidities were included in the regression models as potential confounders. Results were reported as ORs including their respective 95% CIs. P values were reported to describe the statistical significance of the respective independent variable’s influence on the COVID-19 hospitalisation and COVID-19-related death. P values <0.05 were considered significant.
Factors potentially associated with the COVID-19 outcome considered in the model were age, gender, use of TNF inhibitors, no immunomodulatory treatment and regarding the significant differences additionally absence of relevant comorbidities (see tables 1 and 2). Comedication rather than immunomodulatory/immunosuppressive drugs was not recorded in the registry, and therefore, could not be included in the model.
Table 1.
Patient characteristics
| Unvaccinated (reference) | Double-vaccinated | Triple-vaccinated | P value | ||||
| n | % | n | % | n | % | ||
| 923 | 551 | 803 | |||||
| Age (years, median, range) | 55 (18–93) | 55 (20–93) | 56 (16–93) | 0.630* | |||
| Female | 603 | 65.3 | 356 | 64.6 | 576 | 71.7 | 0.005† |
| Body mass index (median, range) | 26.5 (16–53) | 26.7 (17–55) | 25.8 (16–53) | 0.004* | |||
| 1. Vaccine | |||||||
| Moderna | 29 | 5.3 | 56 | 7.0 | |||
| Pfizer/BioNTech | 426 | 77.3 | 573 | 71.4 | |||
| AstraZeneca | 56 | 10.2 | 139 | 17.3 | |||
| Janssen/Johnson&Johnson | 17 | 3.1 | 4 | 0.5 | |||
| Nuvaxovid | 1 | 0.2 | 5 | 0.6 | |||
| Unknown | 22 | 4.0 | 26 | 3.2 | |||
| 2. Vaccine | |||||||
| Moderna | 40 | 7.3 | 66 | 8.2 | |||
| Pfizer/BioNTech | 459 | 83.3 | 644 | 80.2 | |||
| AstraZeneca | 36 | 6.5 | 60 | 7.5 | |||
| Janssen/Johnson&Johnson | 1 | 0.2 | 0 | 0 | |||
| Nuvaxovid | 1 | 0.2 | 7 | 0.9 | |||
| Unknown | 14 | 2.5 | 26 | 3.2 | |||
| 3. Vaccine | |||||||
| Moderna | 201 | 25.0 | |||||
| Pfizer/BioNTech | 573 | 71.4 | |||||
| AstraZeneca | ‡ | 0.4 | |||||
| Janssen/Johnson&Johnson | † | 0.2 | |||||
| Nuvaxovid | 0 | 0 | |||||
| Unknown | 24 | 3.0 | |||||
| Time between SARS-CoV-2 infection and last vaccination (days, median, range) | 151 (14–347) | 88 (14–270) | |||||
| IRD (multiple selection possible) | |||||||
| Rheumatoid arthritis | 393 | 42.6 | 240 | 43.6 | 331 | 41.2 | 0.681† |
| Spondyloarthritis | 278 | 30.2 | 161 | 29.4 | 252 | 31.4 | 0.719† |
| Connective tissue diseases | 132 | 14.3 | 74 | 13.4 | 116 | 14.2 | 0.856† |
| Other vasculitides | 60 | 6.5 | 28 | 5.1 | 45 | 5.6 | 0.499† |
| ANCA-associated vasculitis | 25 | 2.7 | 19 | 3.4 | 20 | 2.5 | 0.561† |
| Other type of IRD | 93 | 10.1 | 51 | 9.3 | 74 | 9.2 | 0.798† |
| Immunomodulation (multiple selection possible) | |||||||
| Glucocorticoids | 247 | 26.8 | 134 | 24.3 | 194 | 24.2 | 0.392† |
| Prednisolone ≤5 mg/day | 169 | 83 | 121 | ||||
| Prednisolone 6–9 mg/day | 10 | 5 | 7 | ||||
| Prednisolone ≥10 mg/day | 33 | 18 | 26 | ||||
| Methotrexate, leflunomide, sulfasalazine | 461 | 49.9 | 258 | 46.8 | 366 | 45.6 | 0.176† |
| Azathioprine | 22 | 2.4 | 16 | 2.9 | 28 | 3.5 | 0.395† |
| Tumour necrosis inhibitors (adalimumab, certolizumab, infliximab, golimumab, etanercept) | 192 | 20.8 | 125 | 22.7 | 197 | 24.5 | 0.180† |
| Other cytokine inhibitors (IL-6/IL-1/IL12/23/IL17 inhibition) | 81 | 8.8 | 68 | 23.8 | 79 | 9.8 | 0.086† |
| Janus kinase inhibitors (baricitinib, tofacitinib, upadacitinib, filgotinib) | 70 | 7.6 | 51 | 9.3 | 71 | 8.8 | 0.468† |
| Immunsuppressives (cyclosporine, mycophenolate, cyclophosphamide) | 28 | 3.0 | 14 | 2.5 | 20 | 2.5 | 0.766† |
| Abatacept | 10 | 1.1 | 8 | 1.5 | 10 | 1.2 | 0.824† |
| Rituximab | 28 | 3.0 | 24 | 4.4 | 35 | 4.4 | 0.270† |
| Belimumab | 8 | 0.9 | 7 | 1.3 | 7 | 0.9 | 0.704† |
| Immunoglobulins | ‡ | 0.3 | 1 | 0.2 | 7 | 0.9 | 0.191‡ |
| Apremilast | 4 | 0.4 | 2 | 0.4 | ‡ | 0.4 | 1.000‡ |
| Other immunmodulators (eg, colchicine) | 51 | 5.5 | 18 | 3.3 | 29 | 3.6 | 0.057† |
| No immunmodulation | 100 | 10.8 | 44 | 8.0 | 57 | 7.1 | 0.018† |
| Disease activity | |||||||
| No/low | 780 | 84.5 | 478 | 86.8 | 710 | 88.4 | 0.059† |
| Moderate/high | 129 | 14.0 | 65 | 11.8 | 78 | 9.7 | 0.024† |
| Comorbidities (multiple selections possible) | |||||||
| Arterial hypertension | 287 | 31.1 | 172 | 31.2 | 229 | 28.5 | 0.428† |
| Other relevant comorbidities | 168 | 18.2 | 110 | 20.0 | 130 | 16.2 | 0.197† |
| Heart failure/arrhythmia | 93 | 10.1 | 67 | 12.2 | 68 | 8.5 | 0.084† |
| Diabetes mellitus | 83 | 9.0 | 50 | 9.1 | 47 | 5.9 | 0.028† |
| Osteoporosis | 52 | 5.6 | 35 | 6.4 | 74 | 9.2 | 0.011† |
| Chronic renal failure | 47 | 5.1 | 29 | 5.3 | 48 | 6.0 | 0.704† |
| Cancer/history of cancer | 26 | 2.8 | 19 | 3.4 | 22 | 2.7 | 0.719† |
| Chronic obstructive lung diseases | 22 | 2.4 | 12 | 2.2 | 24 | 3.0 | 0.597† |
| Interstitial lung diseases | 23 | 2.5 | 12 | 2.2 | 18 | 2.2 | 0.909† |
| Bronchial asthma | 39 | 4.2 | 27 | 4.9 | 42 | 5.2 | 0.607† |
| Pregnancy | 9 | 1.0 | 4 | 0.7 | † | 0.2 | 0.159‡ |
| Pulmonal hypertension | 7 | 0.8 | 2 | 0.4 | † | 0.2 | 0.340‡ |
| Liver cirrhosis | 4 | 0.4 | 1 | 0.2 | ‡ | 0.4 | 0.821‡ |
| Absence of relevant comorbidities | 350 | 37.9 | 250 | 45.4 | 371 | 46.2 | 0.001† |
| COVID-19-associated complications | |||||||
| Hospitalisation | 142 | 15.4 | 44 | 8.0 | 22 | 2.7 | <0.001† |
| Invasive ventilation | 25 | 2.7 | 11 | 2.0 | 1 | 0.1 | <0.001‡ |
| Relevant complications (eg, concomitant infection, thromboembolic events) | 57 | 6.2 | 23 | 4.2 | 6 | 0.7 | <0.001† |
| COVID-19-related death | 18 | 2.0 | 10 | 1.8 | 5 | 0.6 | 0.050† |
*Kruskal-Wallis test.
†χ2 test (Pearson).
‡Fisher’s exact test.
IRD, inflammatory rheumatic disease.
Table 2.
Patient characteristics (hospitalised)
| Unvaccinated (reference) | Double-vaccinated | Triple-vaccinated | P value | ||||
| n | % | n | % | n | % | ||
| 142 | 44 | 22 | |||||
| Age (years, median, range) | 65 (19–93) | 65 (28–86) | 67 (41–87) | 0.685* | |||
| Female | 89 | 62.7 | 23 | 52.3 | 18 | 81.8 | 0.065† |
| Body mass index (median, range) | 27.9 (18–46) | 27.6 (19–43) | 26.45 (18–42) | 0.560* | |||
| 1. Vaccine | |||||||
| Moderna | 1 | 2.3 | 2 | 9.1 | |||
| Pfizer/BioNTech | 34 | 77.3 | 16 | 72.7 | |||
| AstraZeneca | 3 | 6.8 | 2 | 9.1 | |||
| Janssen/Johnson&Johnson | 0 | 0 | 0 | 0 | |||
| Nuvaxovid | 0 | 0 | 0 | 0 | |||
| Unknown | 6 | 13.6 | 2 | 9.1 | |||
| 2. Vaccine | |||||||
| Moderna | 3 | 6.8 | 2 | 9.1 | |||
| Pfizer/BioNTech | 35 | 79.5 | 16 | 72.7 | |||
| AstraZeneca | 4 | 9.1 | 0 | 0 | |||
| Janssen/Johnson&Johnson | 0 | 0 | 0 | 0 | |||
| Nuvaxovid | 0 | 0 | 1 | 4.5 | |||
| Unknown | 2 | 4.5 | 3 | 13.6 | |||
| 3. Vaccine | |||||||
| Moderna | ‡ | 13.6 | |||||
| Pfizer/BioNTech | 14 | 63.6 | |||||
| AstraZeneca | 0 | 0 | |||||
| Janssen/Johnson&Johnson | 0 | 0 | |||||
| Nuvaxovid | 0 | 0 | |||||
| Unknown | 5 | 22.7 | |||||
| Time between SARS-CoV-2 infection and last vaccination (days, median, range) | 149.5 (17–307) | 78.5 (22–120) | |||||
| IRD (multiple selection possible) | |||||||
| Rheumatoid arthritis | 79 | 55.6 | 18 | 40.9 | 12 | 54.5 | 0.227† |
| Spondyloarthritis | 22 | 15.5 | 6 | 13.6 | 1 | 4.5 | 0.490‡ |
| Connective tissue diseases | 15 | 10.6 | 10 | 22.7 | 4 | 18.2 | 0.091‡ |
| Other vasculitides | 9 | 6.3 | 3 | 6.8 | 1 | 4.5 | 1.000‡ |
| ANCA-associated vasculitis | 14 | 9.9 | 7 | 15.9 | 4 | 18.2 | 0.326‡ |
| Other type of IRD | 13 | 9.2 | 2 | 4.5 | 2 | 9.1 | 0.667‡ |
| Immunomodulation (multiple selection possible) | |||||||
| Glucocorticoids | 72 | 50.7 | 23 | 52.3 | 15 | 68.2 | 0.310† |
| Prednisolone ≤5 mg/day | 53 | 37.3 | 14 | 31.8 | 11 | 50.0 | |
| Prednisolone 6–9 mg/day | 1 | 0.7 | 1 | 2.2 | 0 | 0 | |
| Prednisolone ≥10 mg/day | 12 | 8.5 | 5 | 11 | 1 | 4.5 | |
| Methotrexate, leflunomide, sulfasalazine | 84 | 59.1 | 24 | 54.5 | 10 | 45.5 | 0.457† |
| Azathioprine | 6 | 4.2 | 1 | 2.3 | 0 | 0 | 1.000‡ |
| Tumour necrosis inhibitors (adalimumab, certolizumab, infliximab, golimumab) | 16 | 11.3 | 4 | 9.1 | 3 | 13.6 | 0.833‡ |
| Other cytokine inhibitors (IL-6/IL-1/IL12/23/IL17 inhibition) | 5 | 3.5 | 3 | 6.8 | 2 | 9.1 | 0.124‡ |
| Janus kinase inhibitors (baricitinib, tofacitinib, upadacitinib, filgotinib) | 14 | 9.9 | 5 | 11.4 | 1 | 4.5 | 0.404‡ |
| Immunsuppressives (cyclosporine, mycophenolate, cyclophosphamide) | 4 | 2.8 | 5 | 11.4 | 2 | 9.1 | 0.046‡ |
| Abatacept | 3 | 2.1 | 2 | 4.5 | 2 | 9.1 | 0.109‡ |
| Rituximab | 16 | 11.3 | 15 | 34.1 | 7 | 31.8 | 0.001† |
| Belimumab | 1 | 0.7 | 0 | 0 | 0 | 0 | |
| Immunoglobulins | 1 | 0.7 | 0 | 0 | 1 | 1.0 | |
| Other immunmodulators | 5 | 3.5 | 1 | 2.3 | 0 | 0 | |
| No immunmodulation | 9 | 6.3 | 2 | 4.3 | 0 | 0 | |
| Disease activity | |||||||
| No/low | 111 | 78.2 | 30 | 68.2 | 20 | 90.9 | 0.106† |
| Moderate/high | 21 | 14.8 | 14 | 31.8 | 2 | 9.1 | 0.026‡ |
| Comorbidities (multiple selections possible) | |||||||
| Arterial hypertension | 74 | 52.1 | 26 | 59.1 | 12 | 54.5 | 0.718† |
| Other relevant comorbidities | 47 | 33.1 | 17 | 38.6 | 6 | 27.3 | 0.634† |
| Heart failure/arrhythmia | 34 | 23.9 | 17 | 38.6 | 6 | 27.3 | 0.162† |
| Diabetes mellitus | 31 | 21.8 | 4 | 9.1 | 2 | 9.1 | 0.015‡ |
| Osteoporosis | 14 | 9.9 | 6 | 13.6 | 5 | 22.7 | 0.210† |
| Chronic renal failure | 27 | 19.0 | 7 | 15.9 | 6 | 27.3 | 0.540† |
| Cancer/history of cancer | 8 | 5.6 | 4 | 9.1 | 1 | 4.5 | 0.398‡ |
| Chronic obstructive lung diseases | 5 | 3.5 | 4 | 9.1 | 0 | 0 | 0.379‡ |
| Interstitial lung diseases | 9 | 6.3 | 6 | 13.6 | 3 | 13.6 | 0.149‡ |
| Bronchial asthma | 10 | 7.0 | 1 | 2.3 | 3 | 13.6 | 0.192‡ |
| Pregnancy | 2 | 1.4 | 0 | 0 | 0 | 0 | |
| Pulmonal hypertension | 1 | 0.7 | 1 | 2.3 | 1 | 4.5 | |
| Liver cirrhosis | 2 | 1.4 | 0 | 0 | 0 | 0 | |
| Absence of relevant comorbidities | 21 | 14.8 | 7 | 15.9 | 2 | 9.1 | 0.785‡ |
| COVID-19-associated complications | |||||||
| Invasive ventilation | 25 | 17.6 | 11 | 25.0 | 1 | 4.5 | 0.112‡ |
| Relevant complications (eg, concomitant infection, thromboembolic events) | 50 | 35.2 | 19 | 43.2 | 4 | 18.2 | 0.128‡ |
| COVID-19-related death | 18 | 12.7 | 10 | 22.7 | 5 | 22.7 | 0.182† |
*Kruskal-Wallis test.
†χ2 test (Pearson).
‡Fisher’s exact test.
IRD, inflammatory rheumatic disease.
UV patients were defined as the reference group. Calculations were carried out using SPSS Statistics (IBM SPSS Statistics, V.26.0).
Results
Patient characteristics
Since the beginning of the registry, until July 2022, 5296 cases of SARS-CoV-2 infection were reported. In the observation period (February 2021 until July 2022), 2688 cases were reported. After excluding cases with missing information regarding vaccination status, SARS-CoV-2 reinfection, only single vaccination as well as last vaccination less than 14 days prior to documented SARS-CoV-2 infection, 2314 cases remained for the analysis (UV n=923, DV n=551, TV n=803, QV n=37). Most of the patients were female (UV: 65.3%, DV: 64.6%, TV: 71.7%, QV: 65.9%). The median age was over years 55 (UV, DV) and 56 (TV, table 1, data of QV are included in online supplemental table 1). More than half of the patients had an inflammatory joint disease, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and enteropathic arthritis, followed by connective tissue diseases, vasculitides and other types of IRD. In more than 80% of all cases, no or only low disease activity was reported at the time of SARS-CoV-2 infection (table 1). Immunomodulatory treatment and comorbidities at the onset of SARS-CoV-2 infection are listed in table 1.
rmdopen-2023-002998supp001.pdf (64.3KB, pdf)
Among UV, DV and TV patients, there were neither significant differences regarding the type of IRD nor age (table 1). In addition, no significant differences could be detected regarding immunomodulation (table 1). The proportion of cases without any relevant comorbidity differed significantly (UV: 37.9%, DV: 45.4%, TV: 46.2%, p=0.001).
Vaccines
Most patients received Pfizer/BioNTech vaccine for their first, second and third vaccination (table 1). Novavax’s protein-based vaccine was used in n=6 patients for the second vaccination and in n=8 patients for the third vaccination. All vaccines available in Germany were reported (table 1).
Course of SARS-CoV-2 infection
COVID-19-related hospitalisations were lowest in TV with 2.7% (n=22/803), more common in DV with 8.0% (n=44/551) and highest in UV patients with 15.4% (n=142/923, χ2(p)<0.001). COVID-19-related complications were significantly more frequently reported in UV with 6.2% (n=57/923), followed by 4.2% in DV (n=23/551) and 0.7% in TV (n=6/803, p<0.001). While n=18/923 (2.0%) UV patients died, a lower frequency of deaths was reported in DV (n=10/551, 1.8%) and TV (n=5/803, 0.6%) patients (χ2(p)=0.05).
Patients requiring hospitalisation were in median older compared with non-hospitalised patients, without notable differences regarding vaccination (UV: 65 (19–93) years, DV: 65 (28–86) years, TV: 67 (41–97) years). In DV and TV patients, the use of immunosuppressive medication and rituximab was significantly more often reported than in UV (table 2). Compared with other comorbidities, diabetes mellitus was significant less often reported in DV and TV patients (table 2).
Association of COVID-19 vaccination with COVID-19-related hospitalisation and death
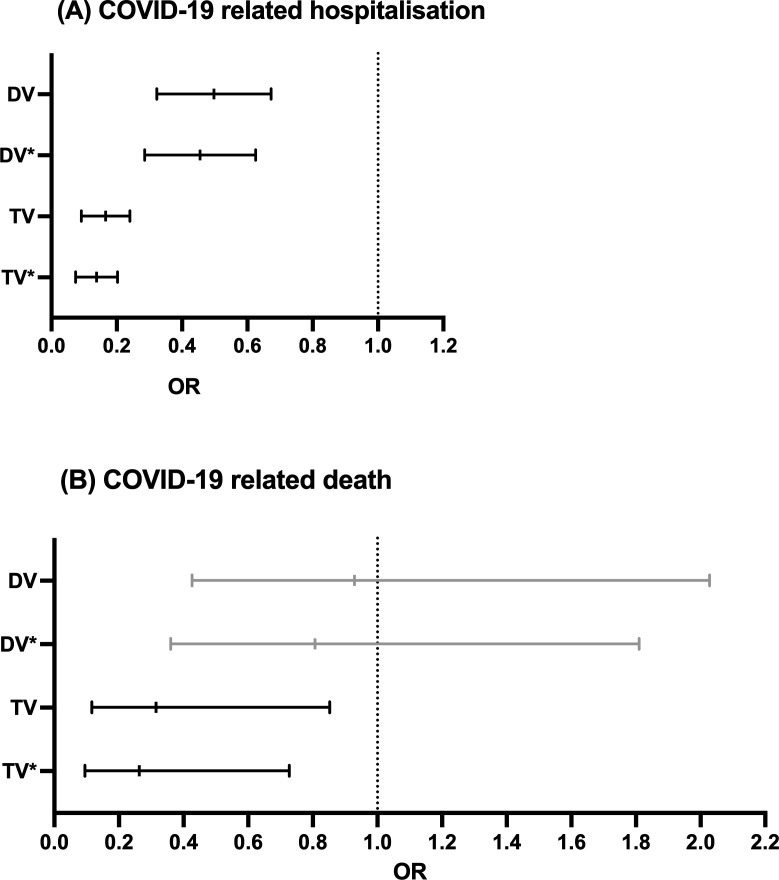
In the univariate logistic regression, DV (OR 0.48, 95% CI 0.33 to 0.68) and TV (OR 0.16, 95% CI 0.10 to 0.25) were significantly associated with a lower risk of hospitalisation (table 3, figure 1A). Multivariable logistic regression, including age, gender, use of TNFi, no immunomodulation and the absence of relevant comorbidities, confirmed a significantly lower risk for hospitalisation in DV (OR 0.43, 95% CI 0.29 to 0.62) and TV (OR 0.13, 95% CI 0.08 to 0.21) patients (table 3, figure 1A).
Table 3.
Logistic regression regarding COVID-19-related hospitalisation
| Double-vaccinated | Triple-vaccinated | Unvaccinated (reference) | |||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Hospitalisation (univariate) |
0.477
(0.334 to 0.682) |
<0.001 |
0.155
(0.098 to 0.245) |
<0.001 | Ref. |
| Hospitalisation (multivariable analysis*) |
0.426
(0.292 to 0.621) |
<0.001 |
0.128
(0.080 to 0.207) |
<0.001 | Ref. |
*Adjusted for age, gender, use of TNF inhibitor, no immunomodulation and absence of any relevant comorbidities.
TNF, tumour necrosis factor.
Figure 1.
COVID-19-related hospitalisation. (B) COVID-19-related death. Results of the univariate and multivariable logistic regression reported as ORs and 95% CIs for the main outcome parameter of this analysis. Unvaccinated patients were defined as reference category. Associations with COVID-19-related hospitalisation are shown in (A) and with COVID-19-related death in (B). (Grey) indicates non-significant data. *Indicates multivariable analyses adjusted for age, gender, use of TNF inhibitor, no immunomodulation and absence of any relevant comorbidities. DV, double vaccinated; TNF, tumour necrosis factor; TV, triple vaccinated.
Regarding COVID-19-related death, in the univariate logistic regression, only TV (OR 0.32, 95% CI 0.12 to 0.85) was significantly associated with a lower risk (table 4, figure 1B). Multivariable logistic regression, including age, gender, use of TNFi, no immunomodulation and the absence of any relevant comorbidities, still revealed a significant lower risk of COVID-19-related death in TV (OR 0.26, 95% CI 0.09 to 0.71) patients (table 4, figure 1B).
Table 4.
Logistic regression regarding COVID-19-related death
| Double-vaccinated | Triple-vaccinated | Unvaccinated (reference) | |||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| COVID-19-related death (univariate) | 0.929 (0.426 to 2.028) |
0.854 |
0.315
(0.116 to 0.852) |
0.023 | Ref. |
| COVID-19-related death (multivariable analysis) | 0.807 (0.360 to 1.810) |
0.602 |
0.263
(0.095 to 0.727) |
0.010 | Ref. |
*Adjusted for age, gender, use of TNF inhibitor, no immunomodulation, and absence of any relevant comorbidities
TNF, tumour necrosis factor.
Discussion
To our knowledge, these are the first data comparing the outcome of breakthrough infections in DV and TV patients with IRD to UV patients with IRD with SARS-CoV-2 infections in a large group of patients with IRD.
Use of immunosuppressives (including rituximab) was significantly more frequently reported in DV and TV patients requiring hospitalisation due to breakthrough infection compared with UV inpatients. This is in accordance with previous studies showing an increased risk of severe COVID-19 regarding the use of rituximab and other immunosuppressives.10–13 Breakthrough infections in DV and TV patients could be due to impaired immune response on vaccination resulting in lower titres of neutralising antibodies against SARS-CoV-2. This occurs especially on B-cell depleting therapy with rituximab. In solid-organ transplant recipients, administration of the third dose of mRNA COVID-19 vaccines resulted in a pronounced increase of antibody titres.14 Likewise, the third dose resulted in seroconversion of 44% of individuals who had remained seronegative on the first two vaccinations.14 Since antibody titres were not part of the data set collected in our registry, no association between breakthrough infections and antibody titres could be retrieved. However, until now, no threshold has been identified for the minimum neutralising antibody titre required for protective immunity. Therefore, studies examining infection rates and antibody titres postvaccination in healthy and in immunosuppressed populations would be necessary.15
In our study, no relevant differences regarding the rate of different types of vaccines could be observed in DV and TV patients. Nevertheless, differences in immune response regarding different type of vaccines could already be observed, which might have in influence on the course of breakthrough infections.16 COVID-19 vaccination involves numerous immune responses such as activation of lymphocytes and producing of neutralising antibodies. However, other pathways such as kinetics involved in cytokines and their receptors, and stimulation of non-T cell responses remain poorly understood. Potential differences depending on the type of vaccines might have an impact on immune response and thus on the outcome of breakthrough infection.17
Our findings support the relevance of triple vaccination for patients with IRD, since in this cohort of patients, triple vaccination was associated with lower rate of hospitalisation and fatal outcome compared with UV. Double vaccination was not significantly associated with lower rate, as in our study only TV patients (n=56) showed a significantly reduced rate of COVID-19-related deaths. Data of the general population were not recorded. But our data are in line with recent published data regarding outcome of breakthrough infection in patients with cancer.18
The strengths of this study are (1) a considerable large number of breakthrough infections in IRDs, (2) the homogenicity of the data that are retrieved from one healthcare system, so all patients received the same standard of medical care and (3) the collection of data by the treating rheumatologists rather than the patients or their relatives. In addition to missing antibody titres, further limitations are (1) a reporting bias which could lead to more severe cases of breakthrough infections, (2) the cross-sectional data collection. It needs to be mentioned that most cases were reported from private practices who performed/organised the vaccinations in conjunction with the general physicians. (3) Despite the large sample size, the results of our study should not be interpreted as incidence rates. We restricted our analysis to confirmed cases of SARS-CoV-2 infection. (4) Although we were able to adjust for several potential confounders in our models, there may still be residual unmeasured confounding. (5) We did not have data available on disease duration or prior medication use, apart from what was reported at the time of SARS-CoV-2 infection. (6) Finally, due to the given caring situations and the German healthcare system, information of type of virus mutation was not available for most of the patients in this dataset. However, using a specific period regarding date of SARS-CoV-2 infection, this might minimise the effect of infection with different type of virus mutation.
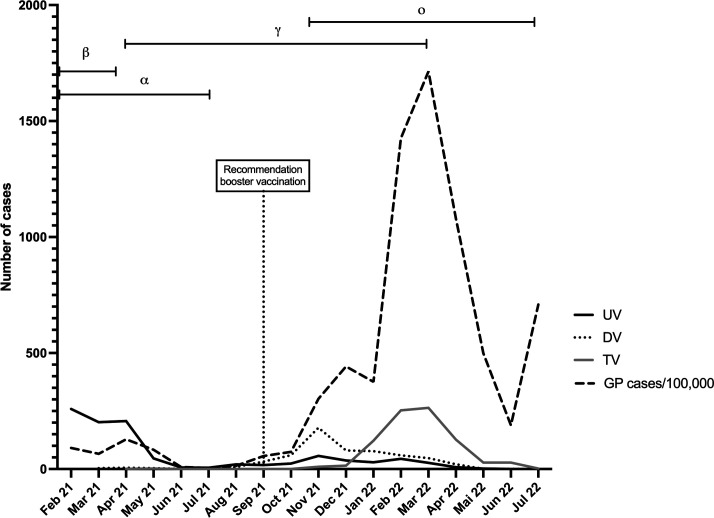
With the beginning of the COVID-19 vaccination campaign which began on 26 December 2020 in Germany, prioritisation rules of specific population groups who should be vaccinated first were established to enable a fair distribution regarding the risk profile of individuals. Depending on the prioritisation rules, patients with IRD younger than 70 years old belonged to group 4 of prioritisations. This group received first vaccine not earlier than spring/summer 2021. As the median age in all three groups was around 55 years, it should be considered that at the beginning of the observation period most patients were UV, and the number of UV patients decreased over time (figure 2). The time between last vaccination and infection might decrease due to the time of recommendation of booster vaccination, which was in September 2021 in Germany. The time course of the pandemic and the number of cases reported in the registry were included in figure 2 to show possible influences of the pandemic on the number of cases depending on the vaccination status in the registry.
Figure 2.
Time course of registry data and number of SARS-CoV-2 infections in the general population in Germany. Number of cases reported in the registry regarding the status of vaccination: unvaccinated (UV), double-vaccinated (DV), triple-vaccinated (TV) and cases of SARS-CoV-2 infection in the general German population (GP) per 100 000 individuals as reported from the Robert-Koch institute. The period of dominance of each virus mutation (α, β, γ, ο) in Germany is shown at the top.
Taken together, our findings support the general recommendation to administer a third dose of SARS-CoV-2 vaccines in patients with IRD.
Acknowledgments
The authors thank Johannes Herrmann (Giessen, Germany) for helping to perform the statistical analysis and clarifying the statement of the manuscript. The authors thank all physicians and personnel involved in the documentation of the cases in our registry.
Footnotes
Twitter: @DocHasseli, @rheuma_doktorin, @ChSpecker
Collaborators: COVID19-Rheuma.de collaborators:Fredrik Albach (Berlin), Annette Alberding (Wuppertal), Tobias Alexander (Berlin), Rieke Alten (Berlin), Susanne Amann (Bad Endbach), Christopher Amberger (Bad Neuenahr), Michaela Amberger (Bad Neuenahr), Bianka Andermann (Berlin), Nils Anders (Braunschweig), Ioana Andreica (Herne), Jan Andresen (Bremen), Nikolaos Andriopoulos (München), Elizabeth Araujo (Erlangen), Peer Aries (Hamburg), Martin Aringer (Dresden), Uta Arndt (Hofheim am Taunus), Sarah Avemarg (Heidelberg), Christoph Baerwald (Leipzig), Erich Bärlin (Ludwigsburg), Nora Bartholomä (Freiburg), Hans Bastian (Berlin-Wannsee), Michael Bäuerle (Bad Aibling), Jutta Bauhammer (Baden-Baden), Christine Baumann (Plauen), Klaus Becker (Blaubeuren), Heidemarie Becker (Münster), Michaela Bellm (Bruchsal), Sylvia Berger (Naunhof), Andrea Berghofen (Mannheim), Gerhard Birkner (Bad Salzuflen), Daniel Blendea (Vreden), Hans Bloching (Bad Aibling), Sebastian Blötz (Erlangen), Christian Blum (Dresden), Stephanie Boeddeker (Marl), Susanne Bogner (Stadtbergen), Martin Bohl-Bühler (Potsdam), Lara Bohnen (Freiburg), Ilka Bösenberg (Frankfurt), Nicole Böttcher (Hamburg), Matthias Braun (Cuxhaven), Diana Braun (Dresden), Matthias Braunisch (München), Jan Philipp Bremer (Hamburg), Matthias Broll (Wetzlar), Andreas Bruckner (Stuttgart-Bad Cannstatt), Veronika Brumberger (München), Martin Brzank (Hamburg), Sahra Büllesfeld (Bad Neuenahr), Sandra Burger (Berlin-Buch), Gamal Chehab (Düsseldorf), Michaela Christenn (Baden-Baden), Anne Claußnitzer (Berlin), Kirsten de Groot (Offenbach), Elvira Decker (Alsfeld), Frank Demtröder (Dortmund), Jacqueline Detert (Templin), Rainer Dörfler (München), Elke Drexler (Mönchengladbach), Valeria Dudics (Rotenburg), Edmund Edelmann (Bad Aibling), Roman Eder (Deggendorf), Christina Eisterhues (Braunschweig), Joachim Michael Engel (Bad Liebenwerda), Brigitte Erbslöh-Möller (Neunkirchen), Miriam Feine (München), Samantha Ferdinan (Frankfurt), Martin Feuchtenberger (Burghausen), Christoph Fiehn (Baden-Baden), Stephanie Finzel (Freiburg), Rebecca Fischer-Betz (Düsseldorf), Martin Fleck (Bad Abbach), Claudia Franke (Tiefenbach), Stefanie Freudenberg (Meerbusch), Christian Fräbel (Giessen), Petra Fuchs (Augsburg), Regina Gaissmaier (Ulm), Ino Gao (Heidelberg), Oliver Gardt (Bochum), Georg Gauler (Osnabrück), Katrin Geißler (Cottbus), Karolina Gente (Heidelberg), Joachim Georgi (Damp), Jasmin Gilly (Landau), Yannik Gkanatsas (Bad Bramstedt), Cornelia Glaser (Freiburg), Agnes Gniezinski- Schwister (Würselen), Rahel Gold (Kaufering), Norman Görl (Rostock), Ralf Görlitz (Warendorf), Karl-Heinz Göttl (Passau), Beate Göttle (Ludwigshafen), Ricardo Grieshaber Bouyer (Heidelberg), Gisela Grothues (Bremen), Mathias Grünke (Bad Aibling), Janine Günther (Heidelberg), Florian Günther (Bad Abbach), Mirjam Haag (Bamberg), Linda Haas (Trier), Anna Haas-Wöhrle (Koblenz), Denitsa Hadjiski (Baden-Baden), Hildrun Haibel (Berlin), Till Hallmann-Böhm (Braunschweig), Peter Härle (Mainz), Urs Hartmann (Mainz), Charlotte Hasenkamp (Münster), Maura-Maria Hauf (Bietigheim-Bissingen), Matthias Hauser (Neu-Ulm), Nicole Heel (München), Guna-Liane Hein (Herne), Reinhard Hein (Nienburg), Claudia Hendrix (Köln), Karen Herlyn (Lübeck), Walter Hermann (Bad Nauheim), Peter Herzer (München), Andrea Himsel (Rüsselsheim), Paula Hoff (Berlin), Marie-Therese Holzer (Bad Bramstedt), Johannes Hornig (Osnabrück), Melanie Huber (Bad Nauheim), Georg Hübner (Lingen), Ole Hudowenz (Lübeck), Axel Hueber (Nürnberg), Sebastian Hüper (Würzburg), Verena Hupertz (Kaufering), Liam Huppke (München), Elke Iburg (Frankfurt), Annette Igney-Oertel (Reutlingen), Steffen Illies (Scwerte), Annett H Jacobi (Rathenow), Ilona Jandova (Freiburg), Christiane Jänicke (Beeskow), Sebastian T Jendrek (Lübeck), Anne Johannes (Wollbach), Aaron Juche (Berlin-Buch), Arne Jung (Berlin), Sarah Kahl (Hamburg), Ludwig Kalthoff (Bochum), Wiebke Kaluza-Schilling (Mainz), Eleni Kampylafka (Erlangen), Antje Kangowski (Rostock), Kirsten Karberg (Berlin), Dorothee Kaudewith (Heidelberg), Gernot Keyßer (Halle), Nayereh Khoshraftar-Yazdi (Lemgo), Melanie Kihm (Heidelberg), Matthias Kirchgässner (Bruchsal), Matthias Kirrstetter (Deggendorf), Birgit Kittel (Bad Liebenwerda), Christoph Kittel (Dresden), Julia Kittler (Erlangen), Arnd Kleyer (Erlangen), Claudia Klink (Gladbeck), Barbara Knau (Erfurt), Christian Kneitz (Schwerin), Katrin Köchel (Frankfurt), Benjamin Köhler (Ratingen), Christian Konietzko (Paderborn), Peter Korsten (Göttingen), Eric Köster (Trier), Ina Kötter (Bad Bramstedt), Magdolna Kovacs (Burghausen), Nelli Kran (Olsberg), Dietmar Krause (Gladbeck), Gabi Kreher (Fulda), Rene Kreutzberger (Bietigheim-Bissingen), Eveline Krieger-Dippel (München), Klaus Krüger (München), Brigitte Krummel-Lorenz (Frankfurt), Martin Krusche (Berlin), Holger Kudela (Magdeburg), Christoph Kuhn (Karlsruhe), Kerstin Kujath (Rüdersdorf), Thomas Kupka (Altenburg), Reiner Kurthen (Aachen), Rolf Kurzeja (Bad Wildungen), Rolf M Küster (Hamburg-Othmarschen), Peter Kvacskay (Heidelberg), Peter Lamprecht (Lübeck), Sabine Langen (Berlin), Heiko Lantzsch (Frankfurt), Petra Lehmann (München), Nicolai Leuchten (Dresden), Christian Löffler (Kirchheim), Dorothea Longerich-Scheuß (Ratingen), Gitta Lüdemann (Kiel), Vanessa Maerz (Lübeck), Hartmut Mahrhofer (Kirchheim), Ingeborg Maier (Ingolstadt), Karin Manger (Bamberg), Elisabeth Märker-Hermann (Wiesbaden), Anette Märtz (München), Hanin Matar (Homburg), Johannes Mattar (Überlingen), Sebastian Maus (Frankfurt), Ursula Mauß-Etzler (Karlsruhe), Florian Meier (Frankfurt), Adelheid Melzer (Seesen), Hans-Jürgen Menne DortmundWolfgang Merkt (Heidelberg), Helga Merwald-Fraenk (München), Claudia Metzler (Regensburg), Sabine Mewes (Vogelsang-Gommern), Julia Meyer (Weimar), Harriet Morf (Erlangen), Harald Mörtbauer (München), Markus Mortsch (Bad Saulgau), Burkhard Muche (Berlin), Nils Murawski (Neunkirchen), Antoine Murray (München), Jana Naumann (Potsdam), Anabell Nerenheim (Berlin), Joachim Neuwirth (Norderstedt), Phuong Nguyen (Leipzig), Stine Niehus (Lübeck), Martin Nielsen (Berlin-Wannsee), Matthias Noehte (Giessen), Dirk Nottarp (Hanau), Dieter Nüvemann (Rendsburg), Wolfgang Ochs (Bayreuth), Sarah Ohrndorf (Berlin), Jürgen Olk (Mainz), Filiz Özden (Nienburg), Bettina Panzer (Hamburg), Alina Patroi (Karlsruhe), Ulrich Pfeiffer (Wuppertal), Dorothea Pick (Bad Neuenahr), Marta Piechalska (Frankfurt), Matthias Pierer (Leipzig), I Pohlenz (Bad Bramstedt), Mikhail Protopopov (Berlin), Almut Pulla (Meerbusch), Michael Purschke (Stendal), Judith Rademacher (Berlin), Wolf Raub (Münster), Jürgen Rech (Erlangen), Anke Reichelt de Tenorio (Kirchheim), Christiane Reindl (München), Annja Reisch (Nürnberg), Gabriela Riemekasten (Lübeck), Markus Rihl (Traunstein), Viale Rissom (Berlin), Karin Rockwitz (Goslar), Maike Rösel (Münster), Markus Röser (Stuttgart), Christoph Rossmanith (Kempten), Maria Roth-Szadorski (München), Ekkehard Röther (Donaueschingen), Fabian Röther (Donaueschingen), Martin Rudwaleit (Bielefeld), Jasemine Saech (Leverkusen), Oliver Sander (Düsseldorf), Eva Sandrock (Freiburg), Ertan Saracbasi-Zender (Oberhausen), Michael Sarholz (Vreden), Christoph Schäfer (Halle), Kerstin Schäfer (Köln), Martin Scheel (Münster), Magnus Scheibel (Ludwigsburg), Stefan Schewe (München), Hermine Schibinger (Augsburg), Andreas Schieweck-Güsmer (Hamburg), Susanne Schinke (Lübeck), Ulrike Schlenker (Kirchheim), Daniel Schlittenhardt (Bad Säckingen), Marc Schmalzing (Würzburg), Matthias Schmitt-Haendle (Bayreuth), Sebastian Schnarr (Bad Füssing), Verena Schneider (Bad Bramstedt), Dieter Schöffel (Mannheim), Michaela Scholz (Mainz), Ulf Schönermarck (München), Jutta Schönherr (Vreden), Ulrich Schoo (Lingen), Anna-Sophie Schübler (Heidelberg), Florian Schuch (Erlangen), Rita Schuck (Neunkirchen), Ulrich Schwab (Kiel), Antje Schwabe (Kahla), Ilka Schwarze (Leipzig), Carola Schwerdt (Dessau), Eva Seipelt (Berlin-Buch), Matthias Sekura (Wesel), Jörg Sensse (Gifhorn), Nyamsuren Sentis (Meerbusch), Christine Seyfert (Breitenbrunn), Ondrej Sglunda (Chemnitz), Naheed Sheikh (Hamburg), Iris Sievert (Hannover), David Simon (Erlangen), Marta Sluszniak (Wuppertal), Katharina Sokoll (Rendsburg), Sigrid Sonn (Stuttgart-Bad Cannstatt), Susanna Späthling-Mestekemper (München), Lydia Sprengler (Radebeul), Gerald Stapfer (Bad Nauheim), Nicolai Steinchen (Kassel), Mirko Steinmüller (Ehringshausen), Karen Steveling (Püttlingen), Karin Stockdreher (Bad Kreuznach), Helga Streibl (Holzkirchen), Johannes Strunk (Köln-Porz), Mechthild Surmann (Münster), Ali Tajali (Püttlingen), Ingo H Tarner (Bad Nauheim), Stefanie Tatsis (Hamburg), Astrid Thiele (Wuppertal), Jan Thoden (Freiburg), Stephan Thrum (Leipzig), Anika Tuleweit (Ludwigshafen), Peter Vaith (Freiburg), Inka Vallbracht-Ackermann (München), Susanne Veerhoff (Hofheim am Taunus), Nils Venhoff (Freiburg), Anita Viardot (Ulm), Lisa Vinnemeier-Laubenthal (Essen), Markus Voglau (Oldenburg), Marcus von Deimling (Freiburg), Cay-Benedict von der Decken (Stolberg), Heike von Löwis (Berlin-Pankow), Marisa Walther (Berlin), Marisa Walther (Berlin), Sven Weidner (Stuttgart), Martin Weigelt (Kyritz), Stefan M Weiner (Trier), Jutta Weinerth (Berlin), Angela Weiß (Dresden), Martin Welcker (Planegg), Stephanie Werner (Düsseldorf), Dirk Wernicke (Hohen Neuendorf), Kristin Wiefel (Dresden), Franziska Wiesent (München), Georg Wiesmüller (Öhringen), Peter Willeke (Münster), Lea Winau (München), Hans Wisseler (Seligenstadt), Matthias Witt (Bad Aibling), Annke Wittig, Stefan Wolf (Sinsheim), Nina Wysocki (Heidelberg), Panagiota Xanthouli (Heidelberg), Monika Zaus (Ingolstadt), Markus Zeisbrich (Freiburg), Zentrum für Rheumatologie (Heidelberg), Silke Zinke (Berlin).
Contributors: RH, JR, UM-L, HS-K and CS: substantial contributions to the conception of the work and the acquisition, analysis and interpretation of data for the work, drafting the work. All authors: substantial contributions to the conception of the work and the interpretation of data for the work. Revising the work critically for important intellectual content, final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
RH, UM-L, CS, JR, and HS-K revised the manuscript. RH, UM-L, CS, JR, and HS-K are responsible for the overall content as the guarantors.
Funding: The registry is funded, in part, by the German Society for Rheumatology.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
COVID19-Rheuma.de collaborators:
Fredrik Albach, Annette Alberding, Tobias Alexander, Rieke Alten, Susanne Aman, Christopher Amberger, Michaela Amberger, Bianka Andermann, Nils Anders, Ioana Andreica, Jan Andresen, Nikolaos Andriopoulos, Elizabeth Arauj, Peer Aries, Martin Aringer, Uta Arndt, Sarah Avemarg, Christoph Baerwald, Erich Bärlin, Nora Bartholomä, Hans Bastian, Michael Bäuerle, Jutta Bauhammer, Christine Baumann, Klaus Becker, Heidemarie Becker, Michaela Bellm, Sylvia Berger, Andrea Berghofen, Gerhard Birkner, Daniel Blendea, Hans Bloching, Sebastian Blötz, Christian Blum, Stephanie Boeddeker, Susanne Bogner, Martin Bohl-Bühler, Lara Bohnen, Ilka Bösenberg, Nicole Böttcher, Matthias Braun, Diana Braun, Matthias Braunisch, Jan Philipp Bremer, Matthias Broll, Andreas Bruckner, Veronika Brumberger, Martin Brzank, Sahra Büllesfeld, Sandra Burger, Gamal Chehab, Michaela Christenn, Anne Claußnitzer, Kirsten de Groot, Elvira Decker, Frank Demtröder, Jacqueline Detert, Rainer Dörfler, Elke Drexler, Valeria Dudics, Edmund Edelmann, Roman Eder, Christina Eisterhues, Joachim Michael Engel, Brigitte Erbslöh-Möller, Miriam Feine, Samantha Ferdinan, Martin Feuchtenberger, Christoph Fiehn, Stephanie Finzel, Rebecca Fischer-Betz, Martin Fleck, Claudia Franke, Stefanie Freudenberg, Christian Fräbel, Petra Fuchs, Regina Gaissmaier, Ino Gao, Oliver Gardt, Georg Gauler, Katrin Geißler, Karolina Gente, Joachim Georgi, Jasmin Gilly, Yannik Gkanatsas, Cornelia Glaser, Agnes Gniezinski- Schwister, Rahel Gold, Norman Görl, Ralf Görlitz, Karl-Heinz Göttl, Beate Göttle, Ricardo Grieshaber Bouyer, Gisela Grothues, Mathias Grünke, Janine Günther, Florian Günther, Mirjam Haag, Linda Haas, Anna Haas-Wöhrle, Denitsa Hadjiski, Hildrun Haibel, Till Hallmann-Böhm, Peter Härle, Urs Hartmann, Charlotte Hasenkamp, Maura-Maria Hauf, and Matthias Hauser
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the ethics committee of the Justus-Liebig-University Giessen (#52–50). Data handling did not involve revealing the identity of any patient. This study was conducted according to the Declaration of Helsinki.
References
- 1. European Medicines Agency . COVID-19 vaccines: key facts. Available: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts#boosters-and-mixing-vaccines-section [Accessed 6 Feb 2022].
- 2. Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-cov-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR coronavirus vaccine (COVAX) physician-reported registry. Ann Rheum Dis 2022;81:695–709.:annrheumdis-2021-221490. 10.1136/annrheumdis-2021-221490 [DOI] [PubMed] [Google Scholar]
- 3. Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-cov-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1351–2. 10.1136/annrheumdis-2021-220656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-cov-2 mrna vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahase E. Covid-19 booster vaccines: what we know and who’s doing what. BMJ 2021;374:2082. 10.1136/bmj.n2082 [DOI] [PubMed] [Google Scholar]
- 6. Connolly CM, Teles M, Frey S, et al. Booster-dose SARS-cov-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis 2022;81:291–3. 10.1136/annrheumdis-2021-221206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanni KM, Patel NJ, DiIorio M, et al. Breakthrough infection after three doses of COVID-19 mrna vaccine in systemic autoimmune rheumatic diseases: two cases in patients on TNF inhibitor monotherapy. RMD Open 2022;8:e002082. 10.1136/rmdopen-2021-002082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liew J, Gianfrancesco M, Harrison C, et al. SARS-cov-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 global rheumatology alliance provider registry. RMD Open 2022;8:e002187. 10.1136/rmdopen-2021-002187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasseli R, Mueller-Ladner U, Schmeiser T, et al. National registry for patients with inflammatory rheumatic diseases (IRD) infected with SARS-cov-2 in germany (recovery): a valuable mean to gain rapid and reliable knowledge of the clinical course of SARS-cov-2 infections in patients with IRD. RMD Open 2020;6:e001332. 10.1136/rmdopen-2020-001332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regierer AC, Hasseli R, Schäfer M, et al. TNFi is associated with positive outcome, but jaki and rituximab are associated with negative outcome of SARS-cov-2 infection in patients with RMD. RMD Open 2021;7:e001896. 10.1136/rmdopen-2021-001896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulze-Koops H, Krueger K, Vallbracht I, et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2021;80:annrheumdis-2020-218075. 10.1136/annrheumdis-2020-218075 [DOI] [PubMed] [Google Scholar]
- 12. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open 2021;4:e2129639. 10.1001/jamanetworkopen.2021.29639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamar N, Abravanel F, Marion O, et al. Three doses of an mrna covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021;385:661–2.:NEJMc2108861. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinson AJ, Anzalone AJ, Sun J, et al. The risk and consequences of breakthrough SARS-cov-2 infection in solid organ transplant recipients relative to non-immunosuppressed controls. Am J Transplant 2022;22:2418–32. 10.1111/ajt.17117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarker P, Akhtar E, Kuddusi RU, et al. Comparison of the immune responses to COVID-19 vaccines in bangladeshi population. Vaccines (Basel) 2022;10:1498. 10.3390/vaccines10091498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kale A, Gaur A, Menon I, et al. An overview of current accomplishments and gaps of COVID-19 vaccine platforms and considerations for next generation vaccines. J Pharm Sci 2023:S0022-3549(23)00021-7. 10.1016/j.xphs.2023.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choueiri TK, Labaki C, Bakouny Z, et al. Breakthrough SARS-cov-2 infections among patients with cancer following two and three doses of COVID-19 mrna vaccines: a retrospective observational study from the COVID-19 and cancer consortium. Lancet Reg Health Am 2023;19:100445. 10.1016/j.lana.2023.100445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-002998supp001.pdf (64.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.