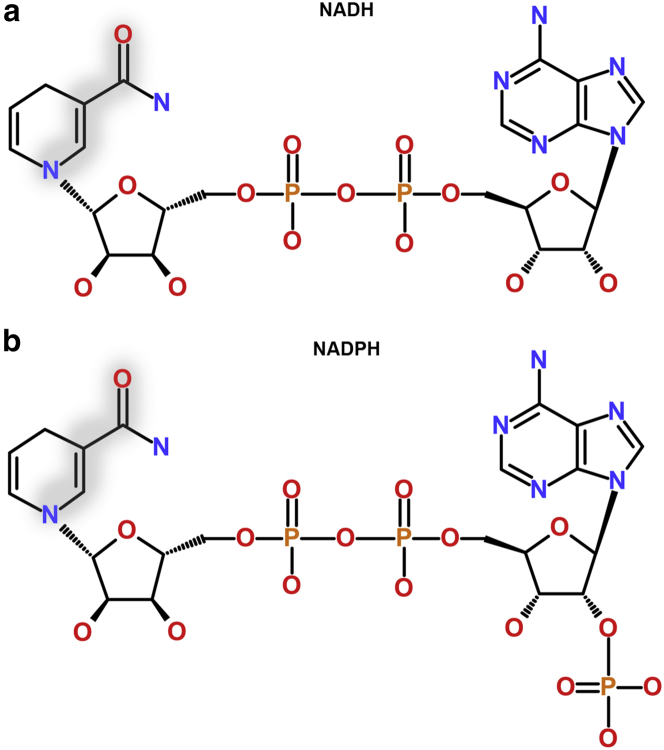
Figure 1.
NADH and NADPH are intrinsically fluorescent, structurally similar enzymatic cofactors that regulate contrasting sets of redox reactions within the cell. Absorption and emission are localized to the nicotinamide ring of both molecules, with the transition involving transfer of charge between the ring nitrogen to the oxygen of the amide group, shown in gray. NADPH (b) differs from NADH (a) only by the presence of a phosphate group at the adenine end of the molecule. Although the distance of this group from the chromophoric region results in identical photophysics of the molecules when isolated in solution, its negative charge allows enzyme binding sites to be specific for either cofactor. This allows NADH to regulate ATP-generating catabolic pathways, whereas NADPH is primarily involved in biosynthesis. To see this figure in color, go online.