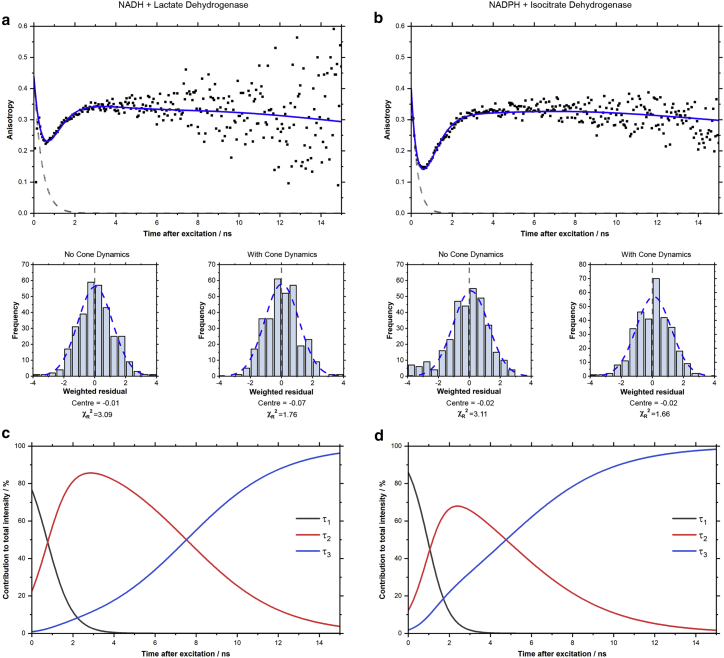
Figure 3.
The dip-and-rise dynamics of the time-resolved fluorescence anisotropies revealed that both longer-lifetime components were associated with slow rotational correlation times. Time-resolved anisotropy datasets were constructed using Eq. 3 and the same polarized fluorescence decays as the time-resolved intensity measurements in Fig. 1 (see Fig. S2). For both (a) NADH with lactate dehydrogenase and (b) NADPH with isocitrate dehydrogenase, acceptable composite anisotropy fits required the introduction of dynamics described by the wobbling-in-a-cone model to the bound components (Eq. 13). These revealed that the shorter-lifetime enzyme-bound component () possessed greater orientational freedom than the longer-lifetime species () in both cofactors. The gray dotted lines in each decay correspond to the anisotropy decay of free NAD(P)H measured in our previous work (22). The time-varying fractional intensities of each component (c and d), calculated using Eq. 12 and the parameters in Table 1, provide fit-free indication that both and are enzyme-bound species, with these components dominating the signal from 2 ns onward and the anisotropy remaining high. To see this figure in color, go online.