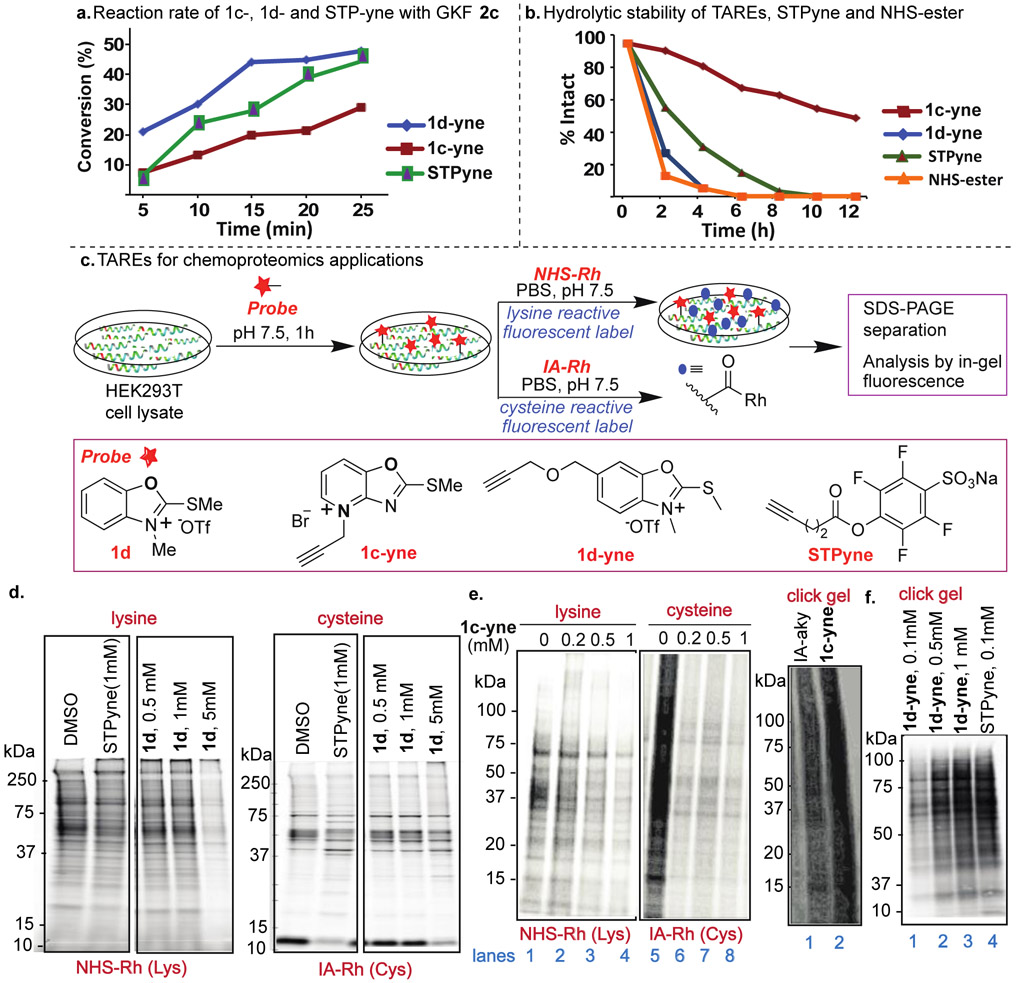
Figure 3.
Chemoproteomic studies of TAREs by gel-based competitive protein profiling. (a) Rate studies with peptide GKF 2c showed high reactivity with 1d-yne. Each time point represents an average of three independent experiments. (b) Hydrolytic stability studies showed 1c-yne is more hydrolytically stable as compared to other lysine reactive probes. (c) General protocol for proteome profiling by different probes and structure of probes. (d) In-gel fluorescence analysis of STPyne (1mM) and 1d probes at different concentrations of 1d (0.5 mM to 5 mM) followed by detection with NHS-Rh for lysine reactivity and IA-Rh for cysteine reactivity. 1d is more selective for lysine as compared to STPyne. (e) In-gel fluorescence analysis of 1c-yne at different concentrations (0 to 1 mM) showed reactivity with both lysine and cysteine. Click gel assay of 1c-yne and cysteine reactive IA-aky probes with cell lysate using fluorescent-biotin azide to determine the total labeled proteins. High labeling of cell lysate was observed with 1c-yne. (f) Click gel assay of 1d-yne at different concentrations (0.1 mM to 1 mM) and STPyne (0.1 mM) probes with cell lysate using biotin-azide and streptavidin blot to determine the total labeled proteins. Dose-dependent labeling was observed for 1d-yne with banding pattern similar to STPyne.