Abstract
Background
Cardiometabolic diseases are highly comorbid, but their relationship with female‐specific or overwhelmingly female‐predominant health conditions (breast cancer, endometriosis, pregnancy complications) is understudied. This study aimed to estimate the cross‐trait genetic overlap and influence of genetic burden of cardiometabolic traits on health conditions unique to women.
Methods and Results
Using electronic health record data from 71 008 ancestrally diverse women, we examined relationships between 23 obstetrical/gynecological conditions and 4 cardiometabolic phenotypes (body mass index, coronary artery disease, type 2 diabetes, and hypertension) by performing 4 analyses: (1) cross‐trait genetic correlation analyses to compare genetic architecture, (2) polygenic risk score–based association tests to characterize shared genetic effects on disease risk, (3) Mendelian randomization for significant associations to assess cross‐trait causal relationships, and (4) chronology analyses to visualize the timeline of events unique to groups of women with high and low genetic burden for cardiometabolic traits and highlight the disease prevalence in risk groups by age. We observed 27 significant associations between cardiometabolic polygenic scores and obstetrical/gynecological conditions (body mass index and endometrial cancer, body mass index and polycystic ovarian syndrome, type 2 diabetes and gestational diabetes, type 2 diabetes and polycystic ovarian syndrome). Mendelian randomization analysis provided additional evidence of independent causal effects. We also identified an inverse association between coronary artery disease and breast cancer. High cardiometabolic polygenic scores were associated with early development of polycystic ovarian syndrome and gestational hypertension.
Conclusions
We conclude that polygenic susceptibility to cardiometabolic traits is associated with elevated risk of certain female‐specific health conditions.
Keywords: cardiometabolic diseases, female health conditions, genomic burden, Mendelian randomization, polygenic risk scores
Subject Categories: Computational Biology, Translational Studies, Big Data and Data Standards
Nonstandard Abbreviations and Acronyms
- eMERGE
Electronic Medical Records and Genomics
- FDR
false discovery rate
- IVW
inverse variance weighted
- LD
linkage disequilibrium
- MR
Mendelian randomization
- PC
principal component
- PMBB
Penn Medicine BioBank
Clinical Perspective.
What Is New?
In this study of >70 000 women from multiple electronic health record–linked biobanks, we evaluated polygenic scores' ability to measure genetic predispositions for a variety of clinically important traits and disorders unique to women.
Using multifactorial approaches for evaluating shared genetic burden and causal relationships, we demonstrate the potential for an inverse causal relationship between coronary artery disease and breast cancer.
We have produced a chronological map of female health including disease prevalence based on shared genetic burden of cardiometabolic traits from adolescence to old age.
What Are the Clinical Implications?
Women with high genetic burden of cardiometabolic conditions may be predisposed to certain diseases such as gestational hypertension, gestational diabetes, and polycystic ovarian syndrome.
Cardiometabolic diseases, such as coronary artery disease (CAD), obesity, hypertension, and type 2 diabetes (T2D) are among the leading causes of death in the world and are highly comorbid. 1 , 2 , 3 Many studies have shown that the pathophysiology of cardiometabolic diseases affects men and women differently. 4 Although cardiometabolic diseases have many sequelae that affect both sexes, including depression, anxiety, chronic obstructive pulmonary disease, and cancer, 5 , 6 they disproportionately affect women because of their links to female‐specific health conditions such as preeclampsia, gestational diabetes, stillbirth, and pregnancy loss. 7 , 8 Multiple lines of evidence link female health conditions to cardiometabolic diseases, where the incidence of cardiometabolic conditions, such as obesity and T2D, leads to associated morbidities. Individuals who develop preeclampsia during pregnancy are more likely to develop cardiovascular diseases and hypertension after pregnancy. 9 , 10 Obesity is closely linked to polycystic ovarian syndrome (PCOS), and individuals with PCOS are at high risk of developing T2D. 11 , 12 , 13 Individuals with endometriosis are at high risk of developing various cancers and cardiovascular diseases such as myocardial infarction and ischemic heart disease. 14 , 15 Despite this evidence, the relationship between female‐specific health conditions and cardiometabolic phenotypes, and particularly the potential for shared genetic risk, is understudied. Investigation of shared genetic risks between cardiometabolic diseases and female‐specific health conditions has the potential to reveal genetic cluster‐based risk factors that can be used to improve screening practices for many diseases in high‐risk patients.
Genome‐wide association studies (GWASs) have exposed common genetic causes among diseases such as T2D, obesity, sleep apnea, hypertension, Alzheimer disease, and many types of cancer. 16 , 17 , 18 , 19 There is evidence of impacts on cardiometabolic phenotypes for >1000 different loci, but the effect size of any single variant is generally minimal. To estimate an individual's overall risk of disease, researchers have used GWAS results to calculate polygenic scores (PGSs), which sum the effects of common single‐nucleotide polymorphisms (SNPs) on a given phenotype 20 and can be included in models predicting the risks of many cardiometabolic phenotypes and comorbidities. 21 , 22
To investigate the effects of genetic risk of cardiometabolic phenotypes on female health conditions, we measured PGSs for cardiometabolic phenotypes and then determined their correlations with female‐specific health conditions documented in electronic health records (EHRs). Prior studies successfully used PGSs as genetic risk factors linking multiple adverse phenotypes. 23 , 24 PGS‐based association tests have the advantage that they are based on unvarying risk factors (ie, inherited genetic burden), and they make fewer assumptions than association tests based on individual genetic factors. We hypothesized that cardiometabolic phenotypes and female‐specific health conditions share common genetic factors so that genetic risks for adverse cardiometabolic phenotypes should associate with female‐specific health conditions. To test this hypothesis, we obtained genotypic and phenotypic data on a wide range of female‐specific health conditions from the PMBB (Penn Medicine BioBank) and the eMERGE (Electronic Medical Records and Genomics) network. We first measured pairwise genetic correlations between cardiometabolic phenotypes and female‐specific health conditions to determine whether there was a common genetic basis. We then estimated the associations between the genetic risks of adverse cardiometabolic phenotypes and female‐specific health conditions. In addition, we evaluated potential causal relationships between cardiometabolic phenotypes and female‐specific health conditions using Mendelian randomization (MR) approaches. Furthermore, because EHR data provide an opportunity to map disease prevalence by age, we generated a chronological map of female‐specific health conditions in individuals with high and low genetic scores to understand age‐specific prevalence in high‐risk and low‐risk individuals.
METHODS
The authors declare that all supporting data for these analyses will be available within the article through its online supplementary files. The authors have individual‐level access to genotype data and medical records from the PMBB and eMERGE network data sets. The eMERGE data set is available for download on dbGAP (phs001584.v2. p2). The PMBB can be accessed upon reasonable request to the corresponding author. The PMBB study was approved by University of Pennsylvania's institutional review board, and the subjects gave informed consent.
Study Populations
Penn Medicine BioBank
The PMBB is a University of Pennsylvania academic biobank that recruits patient participants from the University of Pennsylvania Health System surrounding the greater Philadelphia area in the United States. The PMBB is approved under institutional review board protocol number 813913 and is supported by the Perelman School of Medicine at the University of Pennsylvania. All subjects in the PMBB gave informed consent. The PMBB links patient genotype data with detailed EHR information. Currently, the PMBB contains imputed genotype data for ≈45 000 samples. The PMBB cohort is diverse, with >25% of its participants being of African ancestry. PMBB genotype data were imputed to the The TransOmics for Precesion Medicine (TOPMed) Reference panel using the Michigan Imputation server. We included 21 837 participants from PMBB in this study who self‐reported their sex as women (Table 1). The stratified analyses in this study included only participants of European or African ancestry, whereas those of Asian or Hispanic ancestry were excluded because of sample size limitations. We defined the genetically informed ancestry for PMBB participants by using the kernel density estimation on the principal components.
Table 1.
Sample Sizes in the PMBB and eMERGE Data Sets Overall and Stratified by Ancestry
| Phenotype | PMBB sample size (N cases) | eMERGE sample size (N cases) | ||||
|---|---|---|---|---|---|---|
| All | European | African | All | European | African | |
| Cardiometabolic phenotypes | ||||||
| BMI | 20 209 | 12 344 | 6744 | 39 403 | 32 880 | 5555 |
| CAD | 21 837 (3002) | 13 515 (1956) | 7039 (955) | 49 171 (9597) | 38 918 (7953) | 9067 (1515) |
| DBP | 21 612 | 13 343 | 6994 | NA | NA | NA |
| Hypertension | 21 837 (10278) | 13 515 (5640) | 7039 (4286) | 49 171 (27685) | 38 918 (21883) | 9067 (5265) |
| T2D | 21 837 (4388) | 13 515 (1969) | 7039 (2221) | 49 171 (12403) | 38 918 (8999) | 9067 (3090) |
| Female health phenotypes | ||||||
| Breast cancer | 21 837 (1621) | 13 515 (1146) | 7039 (415) | 49 171 (4148) | 38 918 (3639) | 9067 (443) |
| Cervical cancer | 21 837 (105 | 13 515 (53) | 7039 (50) | 49 171 (332) | 38 918 (263) | 9067 (60) |
| Ectopic pregnancy | 2808 (1779) | 1201 (827) | 1319 (757) | 3078 (823) | 1975 (570) | 641 (172) |
| Endometrial cancer | 21 837 (286) | 13 515 (183) | 7039 (90) | 49 171 (771) | 38 918 (680) | 9067 (83) |
| Endometriosis | 21 837 (701) | 13 515 (320) | 7039 (340) | 49 171 (2314) | 38 918 (1891) | 9067 (354) |
| Excessive fetal growth | 693 (85) | 293 (37) | 329 (42) | 2433 (627) | 1618 (422) | 437 (162) |
| Gestational diabetes | 2655 (523) | 1111 (193) | 1262 (248) | 3174 (762) | 2005 (445) | 719 (251) |
| Gestational hypertension | 2666 (631) | 1118 (256) | 1275 (324) | 3135 (703) | 1980 (411) | 711 (253) |
| Intrauterine death | 685 (57) | 289 (23) | 324 (27) | 2156 (64) | 1438 (44) | 358 (19) |
| Miscarriage | 2934 (389) | 1273 (199) | 1360 (151) | 3568 (823) | 2323 (577) | 740 (200) |
| Ovarian cancer | 21 837 (305) | 13 515 (191) | 7039 (89) | 49 171 (1589) | 38 918 (1359) | 9067 (197) |
| Placenta abruption/previa | 1192 (396) | 482 (157) | 586 (195) | 2674 (997) | 1697 (672) | 583 (262) |
| Polycystic ovarian syndrome | 21 837 (736) | 13 515 (387) | 7039 (272) | 49 171 (1006) | 38 918 (750) | 9067 (209) |
| Poor fetal growth | 792 (202) | 325 (78) | 388 (107) | 2209 (167) | 1478 (115) | 360 (27) |
| Postpartum depression | 924 (384) | 374 (136) | 466 (226) | 2417 (555) | 1579 (349) | 457 (177) |
| Postpartum hemorrhage | 846 (283) | 350 (108) | 408 (146) | 2320 (401) | 1544 (261) | 408 (109) |
| Preeclampsia | 2631 (452) | 1100 (149) | 1264 (272) | 3132 (702) | 1974 (406) | 713 (249) |
| Preterm birth | 687 (66) | 284 (21) | 332 (40) | 2144 (57) | 1433 (35) | 350 (16) |
| Stillbirth | 649 (17) | 270 (3) | 311 (12) | 2123 (8) | 1417 (7) | 347 (0) |
| Uterine cancer | 21 837 (113) | 13 515 (66) | 7039 (43) | 49 171 (418) | 38 918 (348) | 9067 (64) |
| Uterine fibroid | 21 837 (1570) | 13 515 (516) | 7039 (984) | 49 171 (5711) | 38 918 (4103) | 9067 (1455) |
| Vaginal cancer | 21 837 (23) | 13 515 (13) | 7039 (9) | 49 171 (109) | 38 918 (89) | 9067 (18) |
| Vulvar cancer | 21 837 (41) | 13 515 (25) | 7039 (14) | 49 171 (120) | 38 918 (97) | 9067 (21) |
BMI indicates body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; eMERGE, Electronic Medical Records and Genomics; PMBB, Penn Medicine BioBank; and T2D, type 2 diabetes.
Electronic Medical Records and Genomics
The eMERGE network is a nationwide consortium that combines genome‐wide sequence data with EHRs from several health systems across the United States, with most participants coming from the Geisinger Health System and Vanderbilt University. All participants included in eMERGE gave informed consent, and the study was approved by each institute's respective institutional review board. eMERGE data were imputed to the Haplotype Reference Consortium panel using the Michigan Imputation server. Like the PMBB cohort, the eMERGE cohort is diverse across ancestries (≈20% samples of non‐European ancestry) and ages. This study included data from 49 171 self‐reported female patients in the eMERGE network born after 2001 (Table 1). Low sample sizes of participants of Asian and Hispanic ancestry also limited ancestry‐stratified analyses to include only those of European and African ancestry. The ancestry of eMERGE participants was determined based on the intersection of self‐reported race and principal component‐based k‐means clustering. 25
Statistical Analysis
GWASs of Cardiometabolic Phenotypes
To determine genetic correlations and calculate PGSs, we collected quantitative data on the effects of genetic variants on cardiometabolic phenotypes from several large GWASs. Given the diverse nature of our study population, we obtained the largest publicly available multiancestry GWAS summary statistics for 4 cardiometabolic phenotypes: obesity (measured as body mass index [BMI]), CAD, hypertension (measured as diastolic blood pressure [DBP], systolic blood pressure [SBP], and pulse pressure [PP]), and T2D. Summary statistics and source studies for each phenotype are shown in Table 2.
Table 2.
GWAS Data Sets Used to Calculate Genetic Correlations and Polygenic Scores and the Number of SNPs Used From Each GWAS to Calculate the Corresponding PGS
| Phenotype | Ancestries included | Source | Sample size (N cases) | PMID | No. SNPs included in PGS |
|---|---|---|---|---|---|
| Type 2 diabetes | EUR, AFR, EAS, SAS, HIS | Ref. [26] | 1 407 282 (228 499) | 32 541 925 |
PMBB: 1 023 697 eMERGE: 716 330 |
| Body mass index | EUR, AFR, EAS, SAS, HIS | Ref. [27] | 241 258 | 28 443 625 |
PMBB: 885 143 eMERGE: 614 668 |
| Hypertension (DBP, SBP, PP) | EUR, AFR, EAS, SAS, HIS | Ref. [28] | 318 891 | 30 578 418 |
PMBB: 1 024567 eMERGE: 715 471 |
| Coronary artery disease | EUR, AFR, EAS, SAS, HIS | Ref. [29] | 547 261 (122 733) | 29 212 778 |
PMBB: 981 480 eMERGE: 681 029 |
AFR indicates African ancestry; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome wide Replication and Meta‐analysis (CARDIoGRAM) pus The Coronary Artery Disease )C4D) Genetics Consortium; DBP, diastolic blood pressure; EAS, East Asian ancestry; eMERGE, Electronic Medical Records and Genomics; EUR, European ancestry; GIANT, Genetic Investigation of Anthropometric Traits; GWAS, genome‐wide association study; HIS, Hispanic ancestry; MVP, Million Veterans Program; PGS, polygenic score; PMBB, Penn Medicine BioBank; PP, pulse pressure; SAS, South Asian ancestry; SBP, systolic blood pressure; SNPs, single‐nucleotide polymorphisms; and UKBB, UK BioBank.
Genome‐Wide Associations of Female‐Specific Health Conditions
We included 23 female‐specific health conditions to evaluate in this study, including breast cancer, which can affect both sexes but is far more prevalent in women than men. Large multiancestry GWASs are not available for most of these conditions. Therefore, we used PLINK (version 1.90) to conduct GWASs of female‐specific health conditions documented in the PMBB and eMERGE data sets. 30 We filtered the genetic variants in the PMBB and eMERGE imputed data sets to include only variants with imputation quality R 2>0.3 and minor allele frequency >0.01. We then conducted separate GWASs for European and African ancestry individuals in the 2 cohorts and combined results from the 2 cohorts using the meta‐analysis command in PLINK.
Genetic Correlation Calculation
We used the GWASs obtained and generated above to calculate pairwise genetic correlations between cardiometabolic phenotypes and female‐specific health conditions using linkage disequilibrium score regression. 31 Linkage disequilibrium score accounts for linkage disequilibrium (LD) among SNPs by using an external reference panel that should match the ancestry distribution of the corresponding GWASs. We generated European and African ancestry LD reference panels using the HapMap3 SNPs (≈1 M common variants) from all members included in the respective 1000 Genomes population. 32 We then used these LD reference panels to calculate the genetic correlation separately in European and African ancestry participants. We then combined correlation results across ancestries through a meta‐analysis using the metafor R package under the restricted maximum likelihood model.
Polygenic Scores
PGSs calculated using a GWAS of 1 particular ancestry group tend to perform poorly when applied to individuals from a different ancestry group. 20 , 33 , 34 To accurately calculate PGSs for our diverse cohorts, we calculated PGSs for the cardiometabolic phenotypes using the same publicly available GWAS used in the genetic correlation analyses. Weights for each SNP were calculated using PRS‐CS (version from April 24, 2020), a method that performs Polygenic Prediction via Bayseian Regression and Continous Shrinkage Priors. 35 PRS‐CS requires a reference panel that matches the ancestry distribution of the target data set. We generated multiple reference panels for analyses: European‐only reference panel from 1000 Genomes European ancestry population, African‐only reference panel from 1000 Genomes African ancestry population, and a multiancestry LD reference panel using the HapMap SNPs from the entire 1000 Genomes populations (2504 individuals). We identified LD patterns within the 1000 Genomes population by using PLINK (version 1.90) to determine LD blocks and calculate the LD between the SNPs in each block. For PRS‐CS, the global shrinkage parameter φ was fixed to 0.01, and default values were selected for all other parameters. PGSs were then calculated using the weights with PLINK. Only the SNPs in the target data set, summary statistics, and LD reference panel were included in the PGSs. The numbers of SNPs used for each PGS calculation are listed in Table 2. The scores were then normalized (mean of 0 and standard deviation of 1) for each analysis separately (stratified by ancestry and overall).
To evaluate the power of the PGSs, we tested their performance on association of the corresponding primary phenotypes in the summary statistics. We could not obtain quantitative measurements of blood pressure for all participants in the eMERGE cohort, and PP and SBP measurements were not curated in the PMBB cohort. Therefore, we evaluated the performance of the PGSs for blood pressure traits (SBP, DBP, PP) in the eMERGE cohort based on hypertension case–control phenotypes, and we evaluated the performance of the PGS blood pressure traits in the PMBB cohort based on DBP or hypertension (for SBP and PP) as outcomes. We constructed logistic regression models for binary phenotypes (CAD, T2D, and hypertension) and evaluated PGS performance by the area under the receiver operating curves using the pROC package in R. Similarly, we constructed linear regression models for continuous phenotypes (BMI and DBP) and evaluated them by the R 2 using the glm function in R. We also evaluated the value of including PGS in our models by using the likelihood ratio test to compare the null (covariate only) model with the full (PGS and covariates) model using the lmtest package in R. The regression models used birth year and the first 5 principal components (PCs) as covariates. PCs for PMBB and eMERGE were determined from projection onto the 1000 Genomes population. We tested PGS performance overall as well as for subgroups of European or African ancestry. In addition, we constructed European and African LD reference panels using individuals from the respective ancestry groups from 1000 Genomes and computed PGSs using these ancestry‐specific LD panels. We then compared the performance of the PGSs with the PGSs generated using the multiancestry LD reference panel in the PMBB cohort.
Phenotype Data
Cases and controls for each phenotype were defined using International Classification of Diseases (ICD‐9 and ICD‐10) diagnosis codes. Participants were coded as cases of a given phenotype if their records contained at least 1 of the corresponding ICD‐9 or ICD‐10 codes. For pregnancy‐related phenotypes, participants were only considered controls if their records had at least 1 pregnancy‐related ICD‐9 or ICD‐10 code and no ICD‐9 or ICD‐10 codes for relevant complications (such as miscarriage) during pregnancy. Participants were counted as controls for cardiometabolic phenotypes and all non–pregnancy‐related health conditions if their records did not contain any relevant ICD‐9 or ICD‐10 code. The complete list of ICD‐9 and ICD‐10 codes used to include or exclude participants as cases and controls can be found in Table S1. Using these definitions, we determined the sample size for each phenotype in the eMERGE and PMBB cohorts (Table 1).
PGS Association Analysis
We tested the association between each cardiometabolic PGS and female‐specific health condition by fitting separate logistic regression models adjusted by birth year and the first 5 PCs. We conducted this analysis for all participants and for subsets of participants of European or African ancestry. To account for biases from multiple hypothesis testing, we determined if associations passed a Benjamini‐Hochberg false discovery rate (FDR) significance threshold of 0.05, adjusting the P values with the number of hypotheses tested (6 cardiometabolic PGSs×23 female‐specific health conditions=138 hypotheses). The logistic regressions were performed using the glm function in R. The results from the PMBB and eMERGE cohorts were meta‐analyzed using the rma function from the metafor R package under the restricted maximum‐likelihood estimator model. 36 We used PheWAS‐View to visualize the results. 37 We then created prevalence plots for each significant association. The participants were divided into quintiles based on the PGS for each condition, and the percentage of cases was calculated for each quintile.
Mendelian Randomization
To identify evidence of causality between cardiometabolic phenotypes and female‐specific health conditions, we performed 1‐sample MR for 27 significant associations from the PGS analyses using the ivreg function in the ivpack R package and tested which relationships were significant after Benjamini‐Hochberg FDR correction (adjusting for 27 hypotheses). Cardiometabolic PGSs we calculated before were used as genetic instruments, because effect sizes for each SNP were adjusted according to the significance of the association through PRS‐CS. We also included birth year and the first 5 PCs as covariates. The results were then combined in a meta‐analysis using the metafor R package under the restricted maximum‐likelihood estimator model. Because the small sample size for some of the conditions could limit the power of the analysis, we also performed 2‐sample MR for the same associations using the inverse variance weighted (IVW) method in the twoSampleMR package in R. 38 MR sensitivity analyses were conducted using the weighted median and MR Egger methods in the same package, and tests for horizontal pleiotropy were also performed using MR Egger. The genetic instruments for the cardiometabolic phenotypes in the 2‐sample MR were the genome‐wide significant SNPs (P<5×10−8) in the respective cardiometabolic GWAS used to calculate the PGSs. The SNPs were pruned according to LD patterns among the 1000 Genomes HapMap SNPs (r 2=0.1, kb=250), and the remaining representative SNPs were included in the analysis. Matching genetic instruments for the female‐specific health conditions were obtained from publicly available GWASs through the twoSampleMR package (Table S2). Bidirectional MR was also performed for female‐specific health conditions that had at least 1 genome‐wide significant SNP in the GWAS after pruning (r 2=0.1, kb=250). Because these GWASs for female‐specific health conditions were conducted in only European ancestry populations, we performed 2‐sample MR for only associations that were significant in all participants or participants of European ancestry. Two‐sample MR results were then tested for significance after multiple hypothesis correction using an FDR threshold of 0.05 (adjusting for 13 unique significantly associated female‐specific health conditions). Genetic instrument strength for each analysis was calculated using the mean F statistic (β2/σ2) across all SNPs from the cardiometabolic GWASs included in the MR.
Chronology Analyses
We divided the participants into high‐risk and low‐risk groups according to each cardiometabolic PGS. High PGS was defined as the top quintile (>80th percentile), and low PGS was defined as the bottom quintile (<20th percentile). We considered age at the first occurrence of each female‐specific health condition from the ICD‐9 or ICD‐10 records. For pregnancy‐related conditions, the participants were split into 3 age groups: <25, 25 to 39, and 40 to 55 years. We excluded participants who were >55 years of age at the first occurrence of a pregnancy‐related condition because of low sample sizes and potential errors in diagnosis coding. For all nonpregnancy related conditions, the participants were split into 5 age groups: <25, 25 to 39, 40 to 54, 55 to 69, and ≥70 years. We then examined the combined case prevalence of each health condition within the high and low PGS groups in each cohort across all age groups.
RESULTS
Genetic Correlations Among Cardiometabolic Phenotypes and Female‐Specific Health Conditions
We calculated heritability estimates for 13 female‐specific health conditions and proceeded with the genetic correlation analysis for these conditions. We found 10 correlations between the cardiometabolic phenotypes and 6 unique female‐specific health conditions that were at least nominally significant (P<0.05) after meta‐analysis of ancestry‐specific results. (Figure 1A). Two correlations were FDR significant: SBP positively with gestational hypertension (R g=0.292, P=0.0013 [all P values reported in the text are raw P values]) and T2D with PCOS (R g=0.158, P=5.1×10−6). BMI was nominally positively correlated with postpartum depression (R g=0.102, P=0.049), CAD with PCOS (R g=0.148, P=0.0047), PP with gestational hypertension (R g=0.244, P=0.0058) and preeclampsia (R g=0.165, P=0.043), SBP with preeclampsia (R g=0.202, P=0.0089), and T2D with excessive fetal growth (R g=0.0331, P=0.03) and gestational hypertension (R g=0.0711, P=0.014). T2D was also negatively correlated with breast cancer (R g=−0.126, P=0.048). Some other correlations were significant in only 1 ancestry group (Figure S1). For example, CAD was significantly negatively correlated with breast cancer (R g=−0.241, P=0.0013) and T2D was significantly positively correlated with gestational diabetes (R g=0.256, P=4.5×10−9) in the European ancestry‐specific GWASs, and T2D was nominally positively correlated with postpartum depression (R g=0.0773, P=0.035) and postpartum hemorrhage (R g=0.13, P=0.0067) in the African ancestry‐specific GWASs.
Figure 1. Genetic correlation and the influence of shared genetic burden of cardiometabolic traits and health conditions unique to women.
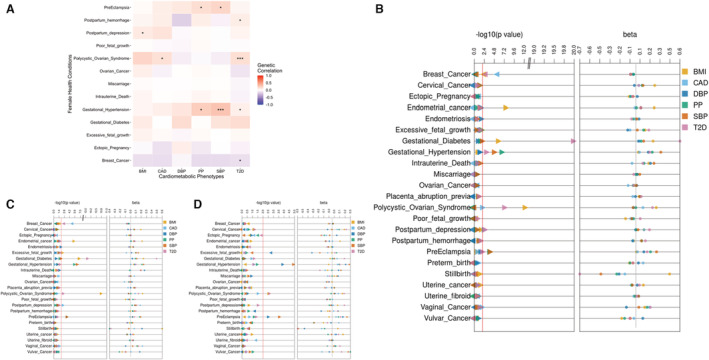
A, Heatmap of genetic correlations between cardiometabolic phenotypes and female‐specific health conditions using multiancestry cardiometabolic genome‐wide association studies (GWASs) and European and African ancestry meta‐analyzed GWASs for female‐specific health conditions. Blue represents negative correlation and red represents positive correlation. Triple asterisks in a box indicate false discovery rate significance, and a single asterisk in a box indicates nominal significance (P<0.05). Genetic correlation was unable to be calculated for grayed‐out boxes. B through D, Results of polygenic score (PGS)‐based meta‐analyses between cardiometabolic PGSs and case–control status of female‐specific health conditions overall (B) and in participants of European (C) and African (D) ancestry. The first panel in these plots corresponds to raw, unadjusted P values, and the second panel shows β estimates. The color of each point refers to the PGS for cardiometabolic traits. The red line corresponds to the P=0.01 threshold. BMI indicates body mass index, CAD, coronary artery disease; DBP, diastolic blood pressure; PP; pulse pressure; SBP, systolic blood pressure; and T2D, type 2 diabetes.
PGS Performance for Predicting Primary Phenotypes
We calculated a PGS for each cardiometabolic phenotype and confirmed the distributions of the raw and normalized scores (Figures S2 through S7). The full model for all PGSs generally performed well in predicting the corresponding primary phenotypes across ancestry groups (Table 3). The covariate‐only (null) model performed better for participants of African ancestry than for participants of European ancestry, whereas the PGS‐only model performed better for participants of European ancestry than for participants of African ancestry. We calculated the difference between the full and null models for all PGSs in European and African ancestry individuals and compared these differences between these 2 groups. The PGSs significantly improved the predictive performance more for participants of European ancestry than for participants of African ancestry (P=0.000488, Wilcoxon signed rank test). Thus, the cardiometabolic PGSs were more accurate in individuals with European ancestry than in individuals with African ancestry, but they improved models in both groups. PGS performance when using different ancestry LD reference panels was similar across phenotypes and ancestry groups (Table S3). Because of relatively more consistent performance and the multiancestry nature of our GWASs and target data sets, we chose to use the PGSs calculated using the multiancestry reference panel for further analyses.
Table 3.
Effect Estimates for Tests of Association of PGS With Primary Phenotype in the eMERGE and PMBB Data Sets
| Phenotype | Ancestry | N total | N cases | R 2 or AUC of full model | β | SE | P value | R 2 or AUC of null model | R 2 or AUC of PGS only model | Likelihood test P value |
|---|---|---|---|---|---|---|---|---|---|---|
| eMERGE | ||||||||||
| BMI | All | 39 338 | 0.08482 | 2.378 | 0.05044 | 0 | 0.0334 | 0.0593 | 0 | |
| EUR | 32 880 | 0.07584 | 1.91 | 0.04046 | 0 | 0.0132 | 0.0622 | 0 | ||
| AFR | 5555 | 0.07118 | 1.585 | 0.1358 | 4.02 E‐31 | 0.0485 | 0.0239 | 3.62 E‐31 | ||
| CAD | All | 49 171 | 9597 | 0.784 | 0.3091 | 0.01318 | 1.62 E‐121 | 0.7745 | 0.5474 | 1.35 E‐123 |
| EUR | 38 918 | 7953 | 0.7732 | 0.3044 | 0.01404 | 2.7 E‐104 | 0.7624 | 0.5629 | 1.07 E‐106 | |
| AFR | 9067 | 1515 | 0.8228 | 0.2161 | 0.03193 | 1.3 E‐11 | 0.8197 | 0.5462 | 1.06 E‐11 | |
| DBP, hypertension | All | 49 171 | 27 685 | 0.8109 | 0.1387 | 0.01347 | 7.17 E‐25 | 0.8099 | 0.529 | 5.99 E‐25 |
| EUR | 38 918 | 21 883 | 0.7994 | 0.1346 | 0.01294 | 2.48 E‐25 | 0.798 | 0.5355 | 1.95 E‐25 | |
| AFR | 9067 | 5265 | 0.8619 | 0.1308 | 0.03028 | 1.56 E‐05 | 0.8612 | 0.5068 | 1.5 E‐05 | |
| PP, hypertension | All | 49 171 | 27 685 | 0.8115 | 0.1644 | 0.01344 | 2.15 E‐34 | 0.8099 | 0.526 | 1.5 E‐34 |
| EUR | 38 918 | 21 883 | 0.8 | 0.1454 | 0.01235 | 5.75 E‐32 | 0.798 | 0.5276 | 3.88 E‐32 | |
| AFR | 9067 | 5265 | 0.8613 | 0.059 | 0.02987 | 0.0484 | 0.8612 | 0.5044 | 0.0483 | |
| SBP, hypertension | All | 49 171 | 27 685 | 0.8131 | 0.2977 | 0.01665 | 1.86 E‐71 | 0.8099 | 0.5352 | 3.97 E‐72 |
| EUR | 38 918 | 21 883 | 0.8023 | 0.253 | 0.01427 | 2.7 E‐70 | 0.798 | 0.5446 | 3.08 E‐71 | |
| AFR | 9067 | 5265 | 0.8622 | 0.1817 | 0.03525 | 2.55 E‐07 | 0.8612 | 0.5031 | 2.36 E‐07 | |
| T2D | All | 49 171 | 12 403 | 0.7193 | 0.6334 | 0.01914 | 3.02 E‐240 | 0.691 | 0.6163 | 1.68 E‐259 |
| EUR | 38 918 | 8999 | 0.6975 | 0.4924 | 0.01515 | 7.23 E‐232 | 0.655 | 0.6145 | 1.18 E‐244 | |
| AFR | 9067 | 3090 | 0.7808 | 0.3786 | 0.03372 | 3.07 E‐29 | 0.7724 | 0.5483 | 9.11 E‐30 | |
| PMBB | ||||||||||
| BMI | All | 20 209 | 0.1542 | 2.658 | 0.08244 | 2.05 E‐222 | 0.1108 | 0.1417 | 1.66 E‐222 | |
| EUR | 12 344 | 0.07977 | 1.9 | 0.06055 | 6.14 E‐208 | 0.006422 | 0.07283 | 4.43 E‐208 | ||
| AFR | 6744 | 0.03556 | 1.696 | 0.122 | 2.46 E‐43 | 0.008037 | 0.2969 | 2.18 E‐43 | ||
| CAD | All | 21 837 | 3002 | 0.8235 | 0.3571 | 0.02354 | 5.73 E‐52 | 0.8148 | 0.5619 | 8.5 E‐53 |
| EUR | 13 515 | 1956 | 0.8303 | 0.3958 | 0.02794 | 1.46 E‐45 | 0.8185 | 0.5929 | 3.13 E‐47 | |
| AFR | 7039 | 955 | 0.799 | 0.1887 | 0.03832 | 8.51 E‐07 | 0.7955 | 0.5389 | 7.89 E‐07 | |
| DBP | All | 21 612 | 0.0425 | 1.036 | 0.06661 | 3.05 E‐54 | 0.03183 | 0.02905 | 2.91 E‐54 | |
| EUR | 13 343 | 0.01555 | 0.7934 | 0.06362 | 1.71 E‐35 | 0.004144 | 0.0124 | 1.62 E‐35 | ||
| AFR | 6994 | 0.04545 | 0.7797 | 0.09169 | 2.23 E‐17 | 0.03571 | 0.0164 | 2.13 E‐17 | ||
| PP, hypertension | All | 21 837 | 10 278 | 0.8125 | 0.1942 | 0.02328 | 7.18 E‐17 | 0.8112 | 0.602 | 5.22 E‐17 |
| EUR | 13 515 | 5640 | 0.7797 | 0.1474 | 0.02008 | 2.11 E‐13 | 0.7778 | 0.5272 | 1.73 E‐13 | |
| AFR | 7039 | 4286 | 0.839 | 0.1205 | 0.03334 | 0.0003 | 0.8383 | 0.5391 | 0.00029 | |
| SBP, hypertension | All | 21 837 | 10 278 | 0.8144 | 0.327 | 0.02523 | 2.08 E‐38 | 0.8112 | 0.6097 | 4.24 E‐39 |
| EUR | 13 515 | 5640 | 0.7817 | 0.2205 | 0.0204 | 5.72 E‐25 | 0.7778 | 0.5397 | 2.84 E‐25 | |
| AFR | 7039 | 4286 | 0.8416 | 0.2763 | 0.03502 | 3.01 E‐15 | 0.8383 | 0.5649 | 1.65 E‐15 | |
| T2D | All | 21 837 | 4388 | 0.754 | 0.8136 | 0.03523 | 5.49 E‐118 | 0.7287 | 0.6713 | 8.59 E‐130 |
| EUR | 13 515 | 1969 | 0.7133 | 0.5425 | 0.02701 | 9.4 E‐90 | 0.6634 | 0.6323 | 5.9 E‐95 | |
| AFR | 7039 | 2221 | 0.7366 | 0.5051 | 0.03602 | 1.13 E‐44 | 0.7168 | 0.5919 | 3.89 E‐46 | |
The β coefficients and SEs are per SD PGS in the full model. Covariates included birth year and the first 5 principal components. AFR indicates African ancestry; AUC, area under the receiver operating curve; BMI, body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; eMERGE, Electronic Medical Records and Genomics; EUR, European ancestry; PGS, polygenic score; PMBB, Penn Medicine BioBank; PP, pulse pressure; SBP, systolic blood pressure; and T2D, type 2 diabetes.
Associations Between Cardiometabolic Risks and Female‐Specific Health Conditions
We detected numerous associations between cardiometabolic PGSs and female health conditions in the meta‐analysis of both cohorts (Figure 1B through 1D). Twenty‐seven associations were statistically significant after correction for multiple hypothesis testing (Table 4). In the meta‐analysis, for all participants and participants of European ancestry, PGSBMI was significantly positively associated with endometrial cancer (βall=0.24, SEall=0.046, P all=9.4×10−8; βeur=0.21, SEeur=0.047, P eur=1.01×10−5) and gestational diabetes (βall=0.23, SEall=0.051, P all=6×10−6; βeur=0.23, SEeur=0.046, P eur=4.4×10−7). PGSBMI was also inversely associated with breast cancer in all participants (βall=−0.071, SEall=0.022, P all=0.0016) and positively associated with PCOS (βall=0.27, SEall=0.039, P all=2.4×10−12). These results suggest that an increased genetic risk of obesity heightens the risks of endometrial cancer, gestational diabetes, and PCOS but decreases the risk of breast cancer. PGSCAD was negatively associated with breast cancer for all participants and participants of European ancestry (βall=−0.072, SEall=0.015, P all=1×10−6; βeur=−0.071, SEall=0.016, P eur=6.5×10−6). This finding suggests that individuals, particularly those of European ancestry, with high PGSCAD, are relatively less likely to have breast cancer compared with individuals with low PGSCAD. PGST2D was significantly positively associated with gestational diabetes in all participants and participants of European ancestry (βall=0.59, SEall=0.063, P all=1.2×10−20; βeur=0.46, SEeur=0.071, P eur=9.4×10−11) and with PCOS in all participants (βall=0.22, SEall=0.043, P all=1.9×10−7). These results support a potential genetic basis for the well‐known associations between T2D and both gestational diabetes and PCOS. 12 , 13 In addition, PGST2D was positively associated in with gestational hypertension in all participants (βall=0.18, SEall=0.064, P all=0.0041) and negatively associated with breast cancer in all participants and participants of European ancestry (βall=−0.073, SEall=0.021, P all=0.00066; βeur=−0.056, SEeur=0.017, Peur=0.0011).
Table 4.
Significant (False Discovery Rate P<0.05) Associations Between Cardiometabolic PGS and Female Health Conditions Identified in the PMBB and eMERGE Meta‐Analysis
| PGS | Association | Ancestry | β | SE | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| BMI | Breast cancer | All | −0.071 | 0.0225 | 0.93 | 0.891–0.973 | 0.00159 |
| Endometrial cancer | All | 0.244 | 0.0458 | 1.28 | 1.17–1.4 | 9.4×10−8 | |
| EUR | 0.206 | 0.0465 | 1.23 | 1.12–1.35 | 1.01×10−5 | ||
| Gestational diabetes | All | 0.23 | 0.0508 | 1.26 | 1.14–1.39 | 6×10−6 | |
| EUR | 0.232 | 0.0458 | 1.26 | 1.15–1.38 | 4.36×10−7 | ||
| PCOS | All | 0.272 | 0.0387 | 1.31 | 1.22–1.42 | 2.37×10−12 | |
| EUR | 0.214 | 0.032 | 1.24 | 1.16–1.32 | 2.13×10−11 | ||
| CAD | Breast cancer | All | −0.0718 | 0.0147 | 0.931 | 0.904–0.958 | 9.96×10−7 |
| EUR | −0.0707 | 0.0157 | 0.932 | 0.904–0.961 | 6.52×10−6 | ||
| DBP | Excessive fetal growth | AFR | −0.313 | 0.0976 | 0.731 | 0.604–0.886 | 0.00135 |
| Gestational hypertension | AFR | 0.204 | 0.0545 | 1.23 | 1.1–1.36 | 0.000179 | |
| PP | Gestational hypertension | All | 0.218 | 0.0443 | 1.24 | 1.14–1.36 | 8.51×10−7 |
| EUR | 0.204 | 0.0452 | 1.23 | 1.12–1.34 | 6.62×10−6 | ||
| Preeclampsia | All | 0.196 | 0.0581 | 1.22 | 1.09–1.36 | 0.000762 | |
| EUR | 0.165 | 0.0486 | 1.18 | 1.07–1.3 | 0.000661 | ||
| SBP | Gestational hypertension | All | 0.332 | 0.0825 | 1.39 | 1.19–1.64 | 5.61×10−5 |
| EUR | 0.233 | 0.0482 | 1.26 | 1.15–1.39 | 1.32×10−6 | ||
| AFR | 0.261 | 0.0605 | 1.3 | 1.15–1.46 | 1.63×10−5 | ||
| Preeclampsia | All | 0.275 | 0.0799 | 1.32 | 1.13–1.54 | 0.000567 | |
| EUR | 0.165 | 0.0486 | 1.18 | 1.07–1.3 | 0.000661 | ||
| AFR | 0.225 | 0.0631 | 1.25 | 1.11–1.42 | 0.000357 | ||
| T2D | Breast cancer | All | −0.0726 | 0.0213 | 0.93 | 0.892–0.97 | 0.000657 |
| EUR | −0.0556 | 0.017 | 0.946 | 0.915–0.978 | 0.00109 | ||
| Gestational diabetes | All | 0.587 | 0.063 | 1.8 | 1.59–2.03 | 1.19×10−20 | |
| EUR | 0.46 | 0.0711 | 1.58 | 1.38–1.82 | 9.37×10−11 | ||
| Gestational hypertension | All | 0.184 | 0.0642 | 1.2 | 1.06–1.36 | 0.00414 | |
| PCOS | All | 0.223 | 0.0428 | 1.25 | 1.15–1.36 | 1.93×10−7 |
P values reported are the raw P values for all associations that are false discovery rate significant. Results were adjusted for birth year and the first 5 principal components. The β coefficients are per SD PGS. BMI indicates body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; eMERGE, Electronic Medical Records and Genomics; EUR, European ancestry; OR, odds ratio; PGS, polygenic score; PCOS, polycystic ovarian syndrome; PMBB, Penn Medicine BioBank; SBP, systolic blood pressure; and T2D, type 2 diabetes.
The 3 PGSs for blood pressure traits (PGSDBP, PGSSBP, and PGSPP) showed varying associations with gestational hypertension and preeclampsia. In the meta‐analysis of both cohorts, PGSDBP was significantly positively associated with gestational hypertension in participants of African ancestry (gestational hypertension: βafr=0.2, SEafr=0.055, P afr=0.00018). PGSDBP was also negatively associated with excessive fetal growth in participants of African ancestry (βafr=−0.31, SEafr=0.098, P afr=0.0014). PGSPP was positively associated with gestational hypertension and preeclampsia in all participants and participants of European ancestry (gestational hypertension: βall=0.22, SEall=0.044, P all=8.5 × 10−7; βeur=0.2, SEeur=0.045, P eur=6.6×10−6; preeclampsia: βall=0.2, SEall=0.058, P all=0.00076; βeur=0.17, SEeur=0.049, P eur=0.00066). PGSSBP was positively associated with gestational hypertension and preeclampsia in all participants, participants of European ancestry, and participants of African ancestry (gestational hypertension: βall=0.33, SEall=0.083, P all=5.6×10−5; βeur=0.23, SEeur=0.048, P eur=1.3×10−6; βafr=0.26, SEafr=0.061, P afr=1.6×10−5; preeclampsia: βall=0.28, SEall=0.08, P all=0.00057; βeur=0.17, SEeur=0.049, P eur=0.00066; βafr=0.23, SEafr=0.063, P afr=0.00036).
For each significant association, we explored the case prevalence of female‐specific health condition per the associated PGS quintile in each cohort (Figure 2A and Figures S8 through S14). The trends matched the results of the association analyses across ancestry groups and in both cohorts. For the positive associations, such as that between PCOS and PGSBMI, the number of cases increased as the PGS percentile increased, whereas the opposite was true for negative associations such as that between breast cancer and PGSCAD (Figure 2A).
Figure 2. Inverse relationship between coronary artery disease (CAD) and breast cancer.
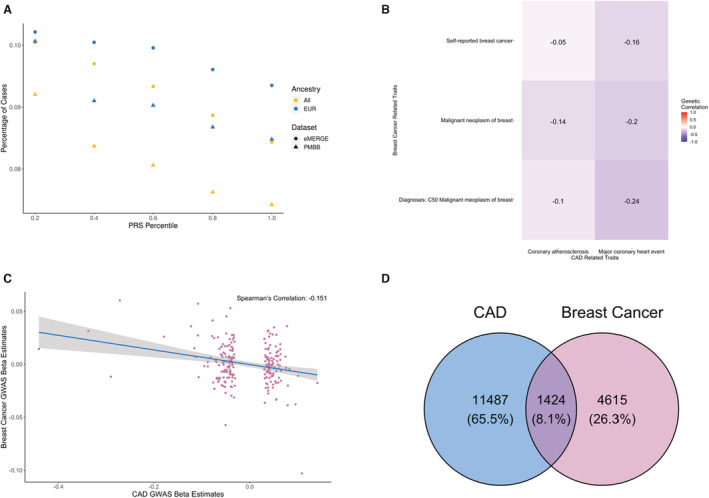
A, Distribution of breast cancer per each polygenic score (PGS)CAD quintile. The x axis represents each PGS quintile, and the y axis represents the disease prevalence. The color of each point refers to the ancestry group (All, European [EUR], and African [AFR]), and the shape indicates the target data set (eMERGE [●] or PMBB [▲]). B, Heatmap of negative genetic correlations between CAD and breast cancer from the UK BioBank Genetic Correlation Browser data set. The gradient of color shows positive (red) to negative (blue) correlations, and the text in each box gives the genetic correlation coefficient. C, Effect estimates of the genome‐wide significant genome‐wide association studies (GWAS) single nucleotide polymorphisms (SNPs) in the CAD GWASs and the corresponding effect estimates of the same SNPs in the breast cancer GWASs. The blue line is the line of best fit from linear regression, and the shaded area around the line is the 95% CI. D, Venn diagram representing the overlap of CAD and breast cancer cases in the PMBB and eMERGE data sets. eMERGE indicates Electronic Medical Records and Genomics; and PMBB, Penn Medicine BioBank.
Association Between PGSCAD and Breast Cancer
To validate the inverse association between PGSCAD and breast cancer, we examined the genetic correlation between CAD and breast cancer using the UK BioBank Genetic Correlation browser (Figure 2B). The genetic correlation between I9_CHD (Major coronary heart disease event) and C50 (Diagnoses‐main ICD‐10: C50 Malignant neoplasm of breast) was significantly negatively correlated (R g=−0.24, P=0.0325). We also compared the effects of SNPs in the CAD GWASs and a large publicly available breast cancer GWAS (Table S2). Among the 220 genome‐wide significant SNPs in the CAD GWASs after pruning, 125 had an opposite effect on breast cancer, and the β estimates of the SNPs were negatively correlated between the 2 GWASs (R g=−0.151, Spearman's correlation) (Figure 2C).
Additionally, to evaluate the risk of ascertainment biases and reporting of comorbidities in the EHR, we assessed the association between PGSCAD and breast cancer in women who were not diagnosed with CAD (n=61 201). We observed a weaker but still negative association between breast cancer and CAD (βall=−0.0481, P all=0.0047; βeur=−0.045, P eur=0.0113) than in the full data set, and this association also remained significant. The overlap between the participants diagnosed with CAD and those diagnosed with breast cancer in our cohorts suggests that women with a diagnosis of CAD were less likely to be diagnosed with breast cancer (Figure 2D).
Mendelian Randomization
We performed 1‐sample MR in the PMBB and eMERGE cohorts for the 27 significant associations and combined them in a meta‐analysis (Figure 3A). The majority of associations were nominally significant (raw P<0.05), and many also remained FDR significant. In all relationships, the direction of the estimated causal effect aligned with the direction of effect in the PGS association analysis. The MR results suggested potential causal relationships between many of the cardiometabolic phenotypes and female health conditions, with the most significant associations being between T2D and gestational diabetes (all: P=1.5×10−11, β=1.52, SE=0.23; EUR: P=4.2×10−11, β=1.7, SE=0.26) and between BMI and PCOS (all: P=1.1×10−8, β=0.0021, SE=0.00037; EUR: P=6.9×10−8, β=0.0021, SE=0.00037).
Figure 3. Mendelian randomization (MR) results for 30 significant polygenic score (PGS)‐based associations.
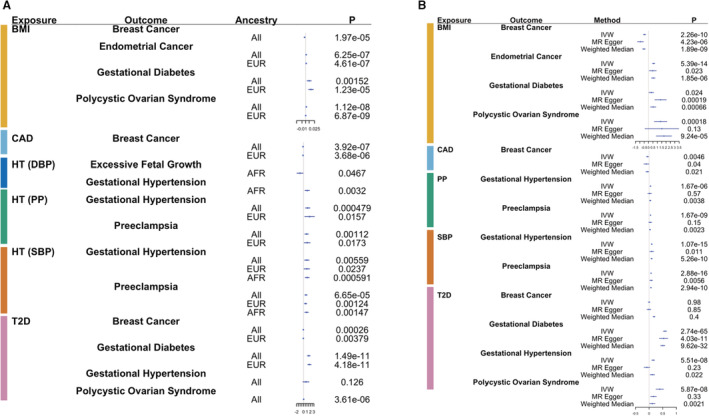
Forest plots show associations between female health conditions as outcomes and cardiometabolic phenotypes as exposures in a 1‐sample (A) and 2‐sample (B) MR analyses. Genetic instruments are the cardiometabolic PGSs in the 1‐sample MR and genome‐wide significant single nucleotide polymorphisms from genome‐wide association studies in the 2‐sample MR. A, Each point refers to β outcome per SD exposure for the body mass index (BMI) PGS (PGSBMI) and log odds ratio (OR) outcome for all other exposures for each test performed as separated by ancestry. B, Each point refers to β outcome/SD exposure for BMI and β outcome/SD log OR exposure for all other variables across all methods used in sensitivity analyses for 2‐sample MR. P values are reported in the last column in both panels. AFR indicates African ancestry; CAD, coronary artery disease; DBP, diastolic blood pressure; EUR, European ancestry; HT, Hypertension; IVW, inverse variance weighted; PP; pulse pressure; SBP, systolic blood pressure; and T2D, type 2 diabetes.
We also performed 2‐sample MR to leverage the power of larger GWAS of female‐specific health conditions (Figure 3B). A few relationships passed FDR significance when using all 3 methods (Table S4), such as T2D and gestational diabetes (IVW: β=0.58, SE=0.034, P=2.74×10−65; MR Egger: β=0.53, SE=0.079, P=4.03×10−11; weighted median: β=0.5, SE=0.042, P=9.62×10−32). Some associations were FDR significant when using the IVW method but became less significant when using the MR Egger and weighted median methods, likely because of the limitations of power of these methods. Other associations were still at least nominally significant (P<0.05) by all 3 methods, such as that between CAD and breast cancer (IVW: β=−0.059, SE=0.021, P=0.0046; MR Egger: β=−0.1, SE=0.049, P=0.04; weighted median: β=−0.064, SE=0.028, P=0.021). Horizontal pleiotropy was only significantly present in our results for BMI and breast cancer (intercept=0.015, SE=0.0056, P=0.0089), BMI and gestational diabetes (intercept=0.35, SE=0.011, P=0.0016), and T2D and gestational hypertension (intercept=0.011, SE=0.0027, P=9.61×10−5). Many relationships with significant pleiotropy still showed at least nominally significant causal effects when accounting for pleiotropy through the MR Egger approach, such as BMI with breast cancer (MR Egger: β=−0.92, SE=0.18, P=4.23×10−6). When performing MR in the opposite direction, we found no relationships that were significant using all 3 methods (Table S5). Some associations were significant when using the IVW and weighted median methods but became insignificant when using MR Egger, such as preeclampsia and SBP (IVW: β=3.46, SE=0.65, P=1.12×10−7; MR Egger: β=7.5, SE=14.9, P=0.7; weighted median: β=3.41, SE=0.41, P=6.06×10−17) and gestational diabetes and T2D (IVW: β=0.2, SE=0.049, P=2.98×10−5; MR Egger: β=0.13, SE=0.11, P=0.26; weighted median: β=0.12, SE=0.011, P=3×10−31). Weak instrument strength could also bias the causal estimate of the exposure on the outcome and its level of significance. The mean F statistics of the SNPs used in each MR analysis were all at least above 57, values that suggest the SNPs included in our analyses were unlikely to be weak and thus do not bias our results (Table S6). Notably, significance and direction of effect remained relatively consistent for most relationships across 1‐ and 2‐sample MR methods.
Role of Population Stratification
Population stratification can confound the results of PGS association and MR analyses. Therefore, we tested the PGS associations with PCs (Table S7). The high R 2 values for most cardiometabolic PGSs indicated that much of the variance in the PGSs could be explained by the PCs, although the R 2 values were smaller when European and African ancestry groups were considered separately. The PCs explained more of the PGS variance for participants of African ancestry than for participants of European ancestry, which suggests that there was more population stratification in the former subpopulation than in the latter, and that there may be confounding factors influencing some results. To overcome these biases, in our prior analyses we accounted for PCs in both the PGS association analyses, used likelihood ratio test to compare the null model with the PGS model, and adjusted with PCs in the 1‐sample MR analyses. Additionally, we performed 1‐sample MR without adjusting for covariates (PCs and birth year) and compared the results with those of the adjusted MR analysis (Table S8). Relationships were generally more significant when using the PGS without including covariates, evidence that further suggests the presence of population stratification in our data sets and the need to adjust for covariates in our models.
Chronology Analyses
We next asked whether genetic risk of cardiometabolic phenotypes affected the time at which female‐specific health conditions developed. We found that participants in the younger age groups with high‐risk cardiometabolic PGSs tended to have more health conditions than those with low‐risk cardiometabolic PGSs (Figure 4 and Figures S15 through S19). In particular, pregnancy‐related conditions were relatively more prevalent in participants <25 years of age with high PGSs than low PGSs for all cardiometabolic PGSs. In addition, participants with low blood pressure PGSs tended to have lower prevalence of gestational hypertension and preeclampsia than other PGSs in the younger age groups (<25 and 25–39 years of age). We observed similar trends for PGST2D and gestational diabetes and for PGSBMI and PCOS. We also observed high prevalence of endometriosis and uterine fibrosis in participants with high‐risk PGSs, particularly those between 25 and 39 years of age.
Figure 4. Chronology analyses of events from the electronic health records.
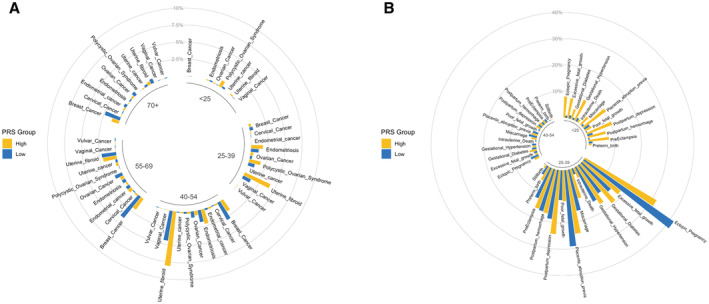
A circular plot shows disease prevalence among participants with high PGSBMI (in yellow) and low PGSBMI (in blue). General female health conditions are shown in (A), and pregnancy and childbirth‐related phenotypes are shown in (B). The circular plots are divided into 5 age categories (<25, 25–39, 40–54, 55–69, and ≥70 years) for general female health conditions and 3 age categories (<25, 25–39, and 40–54 years) for pregnancy‐related phenotypes. BMI indicates body mass index; PGS, polygenic scores; and PRS, polygenic risk score.
DISCUSSION
We observed genetic correlations between cardiometabolic traits and a variety of obstetric and gynecological disorders, and these correlations suggest some significant degree of shared genetic cause among all the phenotypes tested in this study. We investigated the effects of this shared genetic cause by generating PGSs for cardiometabolic phenotypes and testing their associations with various female‐specific health conditions. The PGSs were generally predictive of their corresponding cardiometabolic phenotypes as well as several female‐specific health conditions. Prior research based mainly on nongenetic factors provided evidence for some of these associations, such those between BMI and PCOS and between T2D and gestational diabetes. 11 , 12 Epidemiological studies have also provided evidence of a positive correlation between obesity and endometrial cancer. 39 Our results suggest that certain relationships between cardiometabolic phenotypes and female‐specific health conditions share a genetic basis. Furthermore, our MR results support that cardiometabolic phenotypes have potential causal effects on some female‐specific health conditions.
PGSCAD was inversely associated with breast cancer in individuals of European ancestry. CAD and breast cancer share many common risk factors, such as smoking and poor diet 40 ; however, our results indicated that a high genetic risk of CAD was protective against breast cancer, because participants with high PGSCAD had lower incidence of breast cancer than participants with low PGSCAD. When we considered only participants with high PGSCAD but no CAD diagnosis, we still saw a moderately reduced risk of breast cancer. Several factors might contribute to these results. First, the patterns might reflect ascertainment biases and competing risks. Individuals with high risk of CAD likely live shorter lives than individuals with low risk of CAD and are therefore less likely to be diagnosed with breast cancer during their lifetime. Second, treatments for CAD might protect against breast cancer. Third, genetic mechanisms that predispose individuals to CAD might have protective effects against breast cancer. Our results, together with those of another recent study that showed a protective effect of PGSCAD on breast cancer, suggest that more research should be undertaken to uncover the mechanisms behind this association. 41 Our MR results also suggest a weak but significant negative causal relationship between CAD and breast cancer, and the effect of horizontal pleiotropy for this relationship was also insignificant. In our analyses, we also did not consider breast cancer subtype, which displays differences in key characteristics such as gene expression, disease progression, and treatment response. 42 Breast cancer in our target data sets was defined using only ICD‐9 and ICD‐10 codes and did not consider subtypes, and the breast cancer GWASs we used in this study also included cases of various subtypes. Further research incorporating breast cancer subtype could lead to interesting discoveries about the potential shared genetic factors for CAD and breast cancer.
The 3 blood pressure–related PGSs showed varying degrees of association with hypertensive diseases during pregnancy across ancestry groups. PGSDBP was significantly associated with gestational hypertension and preeclampsia in only participants of African ancestry, whereas only PGSSBP was significantly associated with gestational hypertension and preeclampsia in all participants and participants of European and African ancestry. Prior studies also identified variability in predictive performance when different blood pressure measurements were used to predict hypertension and other diseases. Biases in collecting data from EHRs may have confounded the significance of these associations, and further studies are needed to reach a more definite conclusion on the role blood pressure measurements play in predicting hypertensive diseases during pregnancy. 43 , 44
High genetic risks of most of the cardiometabolic phenotypes tended to associate with higher prevalence of certain health conditions at a young age, even in the absence of an overall association. For example, PGSBMI was not associated with gestational hypertension and ectopic pregnancy overall; however, in the youngest age group (<25 years), these complications were more prevalent in participants with high PGSBMI than in participants with low PGSBMI. Similarly, there was no overall association between PGSSBP and PCOS or endometriosis, but participants who developed PCOS before 25 years of age or endometriosis at 25 to 40 years of age were more likely to have high PGSSBP than low PGSSBP. Patients with high genetic risk of cardiometabolic phenotypes may therefore also have an elevated risk of early development of female‐specific health conditions.
Our study establishes a link between the genetic risks of cardiometabolic phenotypes and several diseases that are unique to women; however, we estimated the genetic risk using PGSs alone, which are based on common variants and do not include the effects of rare variants and copy‐number variations. In addition, we did not consider clinical or environmental factors, such as education level and socioeconomic status. Several studies have shown the effect of nongenetic risk factors on disease risk. 45 , 46 We found that population stratification was potentially present in our cohorts, so we accounted for PCs in our analyses accordingly. However, our focus on PGSs reflects the limitations of incorporating multiple modalities into analyses. Current interest in building integrative risk models suggests that this gap can be closed in the near future. 20 , 47 Furthermore, the weaker performance of PGSs in individuals of African ancestry contributed to a lack of power to identify associations specific to those individuals and suggests a need for future studies to include more racially and ethnically diverse cohorts. We calculated PGS using PRS‐CS with a fixed global shrinkage parameter to reduce computational time, and we chose to set this parameter to 0.01 as recommended by the authors of PRS‐CS for GWASs of smaller sample sizes for highly polygenic traits. Testing different values for the shrinkage parameter may improve the association strength of our PGS and may more accurately identify associations to female‐specific health conditions. The PGSs were also found to have bimodal distributions. In most cases, bimodal distributions are not expected in a population sample, and the bimodal shape could indicate biased sampling in our 2 cohorts for phenotypes associated with the PGSs such as cardiometabolic conditions.
The small sample size for some conditions may have limited the power of our 1‐sample MR analysis. Furthermore, the causal effects identified in our MR analyses might be biased because of horizontal pleiotropy, in which genetic variants associated with cardiometabolic phenotypes affect other traits that in turn influence female‐specific health conditions. We tested for pleiotropy and included methods such as MR Egger that account for pleiotropy and other confounding factors but may not have captured all their effects. Lastly, we also assessed whether our MR analyses was robust to weak instrument bias. Large F statistics (>57) in our analyses reflected the strength of our instrument variable and suggest that the results of our analyses do not suffer from the weak instrument bias.
EHRs are particularly advantageous for investigating disease trajectories and progression. Our analysis provided a visualization of the burden of early diagnosis of female‐specific health conditions in individuals with high genetic risk of cardiometabolic phenotypes. However, these results might have been affected by ascertainment biases of EHRs and inclusion of confounding factors. In addition, because we generated only cardiometabolic PGSs in this study, PGSs specifically calculated for female‐specific health conditions would provide a better understanding on an individual's genetic risk for female‐specific health conditions and the risk for early development of these conditions.
This study illustrates the influence of genetic risk of cardiometabolic phenotypes on female‐specific health conditions and highlight differences in the genetic predisposition among individuals of European and African ancestries. Future studies should incorporate PGSs with other genetic and nongenetic risk factors and study their effects on larger and more diverse multiancestry populations.
Appendix
Regeneron Genetics Center Banner Author List
RGC Management and Leadership Team
Goncalo Abecasis, DPhil; Aris Baras, MD; Michael Cantor, MD; Giovanni Coppola, MD; Andrew Deubler, Aris Economides, PhD; Katia Karalis, PhD; Luca A. Lotta, MD, PhD; John D. Overton, PhD; Jeffrey G. Reid, PhD; Katherine Siminovitch, MD; Alan Shuldiner, MD.
Sequencing and Laboratory Operations
Christina Beechert; Caitlin Forsythe, MS; Erin D. Fuller; Zhenhua Gu, MS; Michael Lattari; Alexander Lopez, MS; John D. Overton, PhD; Maria Sotiropoulos Padilla, MS; Manasi Pradhan, MS; Kia Manoochehri, BS; Thomas D. Schleicher, MS; Louis Widom; Sarah E. Wolf, MS; Ricardo H. Ulloa, BS.
Clinical Informatics
Amelia Averitt, PhD; Nilanjana Banerjee, PhD; Michael Cantor, MD; Dadong Li, PhD; Sameer Malhotra, MD; Deepika Sharma, MHI; Jeffrey Staples, PhD.
Genome Informatics
Xiaodong Bai, PhD; Suganthi Balasubramanian, PhD; Suying Bao, PhD; Boris Boutkov, PhD; Siying Chen, PhD; Gisu Eom, BS; Lukas Habegger, PhD; Alicia Hawes, BS; Shareef Khalid; Olga Krasheninina, MS; Rouel Lanche, BS; Adam J. Mansfield, BA; Evan K. Maxwell, PhD; George Mitra, BA; Mona Nafde, MS; Sean O'Keeffe, PhD; Max Orelus, BA; Razvan Panea, PhD; Tommy Polanco, BA; Ayesha Rasool, MS; Jeffrey G. Reid, PhD; William Salerno, PhD; Jeffrey C. Staples, PhD; Kathie Sun, PhD.
Analytical Genomics and Data Science
Goncalo Abecasis, DPhil; Joshua Backman, PhD; Amy Damask, PhD; Lee Dobbyn, PhD; Manuel Allen Revez Ferreira, PhD; Arkopravo Ghosh, MS; Christopher Gillies, PhD; Lauren Gurski, BS; Eric Jorgenson, PhD; Hyun Min Kang, PhD; Michael Kessler, PhD; Jack Kosmicki, PhD; Alexander Li, PhD; Nan Lin, PhD; Daren Liu, MS; Adam Locke, PhD; Jonathan Marchini, PhD; Anthony Marcketta, MS; Joelle Mbatchou, PhD; Arden Moscati, PhD; Charles Paulding, PhD; Carlo Sidore, PhD; Eli Stahl, PhD; Kyoko Watanabe, PhD; Bin Ye, PhD; Blair Zhang, PhD; Andrey Ziyatdinov, PhD.
Therapeutic Area Genetics
Ariane Ayer, BS; Aysegul Guvenek, PhD; George Hindy, PhD; Giovanni Coppola, MD; Jan Freudenberg, MD; Jonas Bovijn, MD; Katherine Siminovitch, MD; Kavita Praveen, PhD; Luca A. Lotta, MD; Manav Kapoor, PhD; Mary Haas, PhD; Moeen Riaz, PhD; Niek Verweij, PhD; Olukayode Sosina, PhD; Parsa Akbari, PhD; Priyanka Nakka, PhD; Sahar Gelfman, PhD; Sujit Gokhale, BE; Tanima De, PhD; Veera Rajagopal, PhD; Alan Shuldiner, MD; Bin Ye, PhD; Gannie Tzoneva, PhD; Juan Rodriguez‐Flores, PhD.
RGC Biology
Shek Man Chim, PhD; Valerio Donato, PhD; Aris Economides, PhD; Daniel Fernandez, MS; Giusy Della Gatta, PhD; Alessandro Di Gioia, PhD; Kristen Howell, MS; Katia Karalis, PhD; Lori Khrimian, PhD; Minhee Kim, PhD; Hector Martinez; Lawrence Miloscio, BS; Sheilyn Nunez, BS; Elias Pavlopoulos, PhD; Trikaldarshi Persaud, BS.
Research Program Management & Strategic Initiatives
Esteban Chen, MS; Marcus B. Jones, PhD; Michelle G. LeBlanc, PhD; Jason Mighty, PhD; Lyndon J. Mitnaul, PhD; Nirupama Nishtala, PhD; Nadia Rana, PhD.
Sources of Funding
The eMERGE network was initiated and funded by National Human Genome Research Institute through the following grants: Phase IV: U01HG011172 (Cincinnati Children's Hospital Medical Center), U01HG011175 (Children's Hospital of Philadelphia), U01HG008680 (Columbia University), U01HG011176 (Icahn School of Medicine at Mount Sinai), U01HG008685 (Mass General Brigham), U01HG006379 (Mayo Clinic), U01HG011169 (Northwestern University), U01HG011167 (University of Alabama at Birmingham), U01HG008657 (University of Washington Medical Center, Seattle), U01HG011181 (Vanderbilt University Medical Center), U01HG011166 (Vanderbilt University Medical Center serving as the Coordinating Center). Phase III: U01HG8657 (Kaiser Permanente Washington/University of Washington Medical Center), U01HG8685 (Brigham and Women's Hospital), U01HG8672 (Vanderbilt University Medical Center), U01HG8666 (Cincinnati Children's Hospital Medical Center), U01HG6379 (Mayo Clinic), U01HG8679 (Geisinger Clinic), U01HG8680 (Columbia University Health Sciences), U01HG8684 (Children's Hospital of Philadelphia), U01HG8673 (Northwestern University), U01HG8701 (Vanderbilt University Medical Center serving as the Coordinating Center), U01HG8676 (Partners Healthcare/Broad Institute), and U01HG8664 (Baylor College of Medicine).
Disclosures
None.
Supporting information
Tables S1–S8
Figures S1–S18
Acknowledgments
The authors acknowledge PMBB for providing data and thank the patient participants of Penn Medicine who consented to participate in this research program. The authors also thank the PMBB team and Regeneron Genetics Center for providing genetic variant data for analysis. The PMBB is approved under institutional review board protocol number 813913 and is supported by the Perelman School of Medicine at the University of Pennsylvania. The authors also thank the authors of PRS‐CS for providing us with the code needed to generate the multiancestry LD reference panel. Data set: SNPs and their weights used to calculate PGS for each cardiometabolic condition in eMERGE and PMBB data are available in supplementary material to download. B.X. performed the analysis. S.S.V. designed the study. B.X. and S.S.V. wrote the article. T.D. and A.L. helped with data extraction and analyses. H.H., L.K., N.E., W.‐Q.W., and Y.L. helped with data generation. B.X., D.R.V.E., T.D., K.G., B.K., C.W., G.P.J., D.K., M.R., and S.S.V. critically reviewed and approved the article. Regeneron Genetics Center genotyped the Penn Medicine BioBank data set.
Preprint posted on MedRxiv February 04, 2022. doi: https://doi.org/10.1101/2022.02.02.22269844.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.026561
For Sources of Funding and Disclosures, see page 15.
Contributor Information
Shefali Setia Verma, Email: shefali.setiaverma@pennmedicine.upenn.edu.
for the Regeneron Genetics Center:
Goncalo Abecasis, Aris Baras, Michael Cantor, Giovanni Coppola, Andrew Deubler, Aris Economides, Katia Karalis, Luca A. Lotta, John D. Overton, Jeffrey G. Reid, Katherine Siminovitch, Alan Shuldiner, Christina Beechert, Caitlin Forsythe, Erin D. Fuller, Zhenhua Gu, Michael Lattari, Alexander Lopez, John D. Overton, Maria Sotiropoulos Padilla, Manasi Pradhan, Kia Manoochehri, Thomas D. Schleicher, Louis Widom, Sarah E. Wolf, Ricardo H. Ulloa, Amelia Averitt, Nilanjana Banerjee, Michael Cantor, Dadong Li, Sameer Malhotra, Deepika Sharma, Jeffrey Staples, Xiaodong Bai, Suganthi Balasubramanian, Suying Bao, Boris Boutkov, Siying Chen, Gisu Eom, Lukas Habegger, Alicia Hawes, Shareef Khalid, Olga Krasheninina, Rouel Lanche, Adam J. Mansfield, Evan K. Maxwell, George Mitra, Mona Nafde, Sean O’Keeffe, Max Orelus, Razvan Panea, Tommy Polanco, Ayesha Rasool, Jeffrey G. Reid, William Salerno, Jeffrey C. Staples, Kathie Sun, Goncalo Abecasis, Joshua Backman, Amy Damask, Lee Dobbyn, Manuel Allen Revez Ferreira, Arkopravo Ghosh, Christopher Gillies, Lauren Gurski, Eric Jorgenson, Hyun Min Kang, Michael Kessler, Jack Kosmicki, Alexander Li, Nan Lin, Daren Liu, Adam Locke, Jonathan Marchini, Anthony Marcketta, Joelle Mbatchou, Arden Moscati, Charles Paulding, Carlo Sidore, Eli Stahl, Kyoko Watanabe, Bin Ye, Blair Zhang, Andrey Ziyatdinov, Ariane Ayer, Aysegul Guvenek, George Hindy, Giovanni Coppola, Jan Freudenberg, Jonas Bovijn, Katherine Siminovitch, Kavita Praveen, Luca A. Lotta, Manav Kapoor, Mary Haas, Moeen Riaz, Niek Verweij, Olukayode Sosina, Parsa Akbari, Priyanka Nakka, Sahar Gelfman, Sujit Gokhale, Tanima De, Veera Rajagopal, Alan Shuldiner, Bin Ye, Gannie Tzoneva, Juan Rodriguez‐Flores, Shek Man Chim, Valerio Donato, Aris Economides, Daniel Fernandez, Giusy Della Gatta, Alessandro Di Gioia, Kristen Howell, Katia Karalis, Lori Khrimian, Minhee Kim, Hector Martinez, Lawrence Miloscio, Sheilyn Nunez, Elias Pavlopoulos, Trikaldarshi Persaud, Esteban Chen, Marcus B. Jones, Michelle G. LeBlanc, Jason Mighty, Lyndon J. Mitnaul, Nirupama Nishtala, and Nadia Rana
References
- 1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 3. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerdts E, Regitz‐Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–1666. doi: 10.1038/s41591-019-0643-8 [DOI] [PubMed] [Google Scholar]
- 5. Buddeke J, Bots ML, van Dis I, Visseren FL, Hollander M, Schellevis FG, Vaartjes I. Comorbidity in patients with cardiovascular disease in primary care: a cohort study with routine healthcare data. Br J Gen Pract. 2019;69:e398–e406. doi: 10.3399/bjgp19X702725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kendir C, van den Akker M, Vos R, Metsemakers J. Cardiovascular disease patients have increased risk for comorbidity: a cross‐sectional study in The Netherlands. Eur J Gen Pract. 2018;24:45–50. doi: 10.1080/13814788.2017.1398318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and women's health: an evidence‐based review. J Am Board Fam Med. 2011;24:75–85. doi: 10.3122/jabfm.2011.01.100076 [DOI] [PubMed] [Google Scholar]
- 8. Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med. 2019;8:1625. doi: 10.3390/jcm8101625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15:114–121. doi: 10.1007/s11906-013-0329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70:798–803. doi: 10.1161/HYPERTENSIONAHA.117.09246 [DOI] [PubMed] [Google Scholar]
- 11. Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994 [DOI] [PubMed] [Google Scholar]
- 12. Kwak SH, Jang HC, Park KS. Finding genetic risk factors of gestational diabetes. Genomics Inform. 2012;10:239–243. doi: 10.5808/GI.2012.10.4.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, Pasquali R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long‐term prospective study. Diabetes. 2012;61:2369–2374. doi: 10.2337/db11-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9:257–264. doi: 10.1161/CIRCOUTCOMES.115.002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis‐associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience. 2018;12:803. doi: 10.3332/ecancer.2018.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang C, Zhang K, Veluchamy A, Hébert HL, Looker HC, Colhoun HM, Palmer CN, Meng W. A genome‐wide association study provides new evidence that CACNA1C gene is associated with diabetic cataract. Invest Ophthalmol Vis Sci. 2016;57:2246–2250. doi: 10.1167/iovs.16-19332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel SR. Shared genetic risk factors for obstructive sleep apnea and obesity. J Appl Physiol. 1985;2005(99):1600–1606. doi: 10.1152/japplphysiol.00501.2005 [DOI] [PubMed] [Google Scholar]
- 18. Zhuang QS, Zheng H, Gu XD, Shen L, Ji HF. Detecting the genetic link between Alzheimer's disease and obesity using bioinformatics analysis of GWAS data. Oncotarget. 2017;8:55915–55919. doi: 10.18632/oncotarget.19115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rashkin SR, Graff RE, Kachuri L, Thai KK, Alexeeff SE, Blatchins MA, Cavazos TB, Corley DA, Emami NC, Hoffman JD, et al. Pan‐cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nat Commun. 2020;11:4423. doi: 10.1038/s41467-020-18246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 2020;12:44. doi: 10.1186/s13073-020-00742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. Genome‐wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinthorsdottir V, McGinnis R, Williams NO, Stefansdottir L, Thorleifsson G, Shooter S, Fadista J, Sigurdsson JK, Auro KM, Berezina G, et al. Genetic predisposition to hypertension is associated with preeclampsia in European and central Asian women. Nat Commun. 2020;11:5976. doi: 10.1038/s41467-020-19733-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fritsche LG, Gruber SB, Wu Z, Schmidt EM, Zawistowski M, Moser SE, Blanc VM, Brummett CM, Kheterpal S, Abecasis GR, et al. Association of polygenic risk scores for multiple cancers in a phenome‐wide study: results from the Michigan genomics initiative. Am J Hum Genet. 2018;102:1048–1061. doi: 10.1016/j.ajhg.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen X, Howard DM, Adams MJ, Hill WD, Clarke TK, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium , Deary IJ, Whalley HC, AM MI. A phenome‐wide association and Mendelian randomisation study of polygenic risk for depression in UK biobank. Nat Commun. 2020;11:2301. doi: 10.1038/s41467-020-16022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanaway IB, Hall TO , Rosenthal EA, Palmer M, Naranbhai V, Knevel R, Namjou‐Khales B, Carroll RJ, Kiryluk K, Gordon AS, et al. The eMERGE genotype set of 83,717 subjects imputed to ~40 million variants genome wide and association with the herpes zoster medical record phenotype. Genet Epidemiol. 2019;43:63–81. doi: 10.1002/gepi.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, Assimes TL, Lorenz K, Zhu X, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi‐ancestry meta‐analysis. Nat Genet. 2020;52:680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Justice AE, Winkler TW, Feitosa MF, Graff M, Fisher VA, Young K, Barata L, Deng X, Czajkowski J, Hadley D, et al. Genome‐wide meta‐analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat Commun. 2017;8:14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, Torstenson ES, Kovesdy CP, Sun YV, Wilson OD, et al. Trans‐ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genetics. 2019;51:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second‐generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bulik‐Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium , Patterson N, Daly MJ, Price AL, Neale BM. LD score regression distinguishes confounding from polygenicity in genome‐wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. International HapMap 3 Consortium , Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, Peterson R, Domingue B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019;10:3328. doi: 10.1038/s41467-019-11112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1776. doi: 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 37. Pendergrass SA, Dudek SM, Crawford DC, Ritchie MD. Visually integrating and exploring high throughput phenome‐wide association study (PheWAS) results using PheWAS‐view. BioData Mining. 2012;5:5. doi: 10.1186/1756-0381-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR‐base platform supports systematic causal inference across the human phenome. elife. 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke SL, Parham M, Lankester J, Shadyab AH, Liu S, Kooperberg C, Manson JE, Tcheandjieu C, Assimes TL. Broad clinical manifestations of polygenic risk for coronary artery disease in the women's health initiative. Commun Med. 2022;2:108. doi: 10.1038/s43856-022-00171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson KS, Conant EF, Soo MS. Molecular subtypes of breast cancer: a review for breast radiologists. J Breast Imaging. 2021;3:12–24. doi: 10.1093/jbi/wbaa110 [DOI] [PubMed] [Google Scholar]
- 43. Kanegae H, Oikawa T, Okawara Y, Hoshide S, Kario K. Which blood pressure measurement, systolic or diastolic, better predicts future hypertension in normotensive young adults? J Clin Hypertens. 2017;19:603–610. doi: 10.1111/jch.13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. doi: 10.1161/01.HYP.36.5.801 [DOI] [PubMed] [Google Scholar]
- 45. Chatterjee N, Shi J, García‐Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392–406. doi: 10.1038/nrg.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dimovski K, Orho‐Melander M, Drake I. A favorable lifestyle lowers the risk of coronary artery disease consistently across strata of non‐modifiable risk factors in a population‐based cohort. BMC Public Health. 2019;19:1575. doi: 10.1186/s12889-019-7948-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kachuri L, Graff RE, Smith‐Byrne K, Meyers TJ, Rashkin SR, Ziv E, Witte JS, Johansson M. Pan‐cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat Commun. 2020;11:6084. doi: 10.1038/s41467-020-19600-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S18


