Abstract
Background
Transthoracic echocardiography is part of the regular follow‐up protocol at most pediatric pulmonary arterial hypertension (PAH) centers. We aimed to develop a comprehensive and simple echocardiographic risk stratification for children with PAH.
Methods and Results
We included 63 children with PAH and a biventricular cardiac anatomy without relevant shunt lesions (60% female patients; mean age, 9.0 years; 42 idiopathic PAH and 21 associated PAH) undergoing a standardized transthoracic echocardiographic assessment. The prognostic value of echocardiographic parameters was assessed using Cox proportional hazards survival analysis and recursive partitioning for classification tree methods. Over a median follow‐up period of 4.0 years, 17 patients died and 4 underwent lung transplantation. Various echocardiographic parameters were associated with the combined endpoint of death and transplantation on univariate analysis. On further analysis, right atrial area (z score) and left ventricular diastolic eccentricity index (LVEId) emerged as robust and independent predictors of transplant‐free survival. Considering mortality alone as an end point, a combination of right atrial area, left ventricular diastolic eccentricity index, and tricuspid annular plane systolic excursion were identified as independent predictors of outcome. Based on these parameters, we propose simple risk scores that can be applied at the bedside without computer assistance.
CONCLUSIONS
Echocardiographic parameters predict prognosis in children with pulmonary hypertension. A combination of widely available parameters including right atrial area, left ventricular eccentricity index, and tricuspid annular plane systolic excursion emerged as risk stratifiers that await external validation but may assist clinicians determining the prognosis of children with PAH.
Keywords: children, echocardiography, prognosis, pulmonary hypertension
Subject Categories: Congenital Heart Disease, Pulmonary Hypertension, Echocardiography
Nonstandard Abbreviations and Acronyms
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- TAPSE
tricuspid annular plane excursion
- TR
tricuspid regurgitation
Clinical Perspective
What Is New?
Echocardiographic parameters predict prognosis in children with pulmonary arterial hypertension.
A combination of widely available parameters, including right atrial area, left ventricular eccentricity index, and tricuspid annular plane excursion, emerged as a simple risk stratification score that may assist clinicians to determine prognosis of pediatric patients with pulmonary arterial hypertension.
Based on the results, we provide a simple risk stratification pathway that may be useful for clinicians at the bedside.
What Are the Clinical Implications?
While awaiting external validation, the results of the current study should inform recommendations on risk stratification and management in children with pulmonary hypertension.
Pulmonary arterial hypertension (PAH) represents a rare but life‐threatening chronic condition in children. 1 , 2 Despite major advances in drug therapy over the past 3 decades, 3 , 4 , 5 morbidity and mortality are considerable and no definitive cure for the disease exists. 6 The only available treatment option in patients with deteriorating end‐stage disease—despite maximum medical therapy—remains lung transplantation, which in itself is associated with considerable risk and limited survival. 7 Treatment for pediatric PAH is resource intensive and requires special expertise. 2 , 8 A major challenge to treating physicians is the lack of robust risk stratification tools, allowing clinicians to guide therapy and direct high‐risk patients to parenteral prostanoid therapy and consideration of lung transplantation. 9 , 10 While various noninvasive assessment tools exist, transthoracic echocardiography remains the most important modality in the routine regular assessment of patients, including 6‐minute walk test distance and natriuretic peptide levels—. 11 , 12 , 13 , 14 , 15 It allows for the estimation of pulmonary artery pressures (directly or indirectly) and has a central role in assessing ventricular function and providing information on disease progression. 6 , 16 We and others have demonstrated the utility of transthoracic echocardiography for the assessment and management of pediatric patients with PAH. 11 , 14 , 15
The aim of the current study was, based on standardized echocardiographic investigations performed at a major national referral center for pediatric pulmonary hypertension (PH), to evaluate the value of echocardiographic parameters for estimating transplant‐free survival and mortality in children with PAH managed in this specialized, centralized service. Several parameters have been linked to PAH and prognosis, we aimed to delineate the most important parameters amongst them, to develop a simple risk stratification pathway that may be useful for clinicians at the bedside and easily validated externally by other groups.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This was a retrospective analysis of patients with PAH under follow‐up at the UK Pulmonary Hypertension Service for Children, Great Ormond Street Hospital London between 2005 and 2013. This represents an unselected subset of patients subjected prospectively to standardized echocardiographic investigations according to an internal standard protocol. All patients had biventricular cardiac anatomy without relevant shunt lesions. Patients with large intracardiac defects or univentricular circulation were excluded from the analysis. Relevant valvular dysfunction, except for secondary tricuspid or pulmonary regurgitation (which can be regarded as part of PH), also represented exclusion criteria.
Echocardiographic investigations were performed on Vivid 7 machines (GE Healthcare, Milwaukee, WI) using 3.5‐ and 7‐MHz matrix‐array probes. The full echocardiographic protocol, including tissue Doppler echocardiography employed has been described in detail before. 11
Patients were followed from the time of echocardiography, and a composite end point of death or lung transplantation was used to assess the association between echocardiographic parameters and outcome. In addition, a secondary end point of mortality alone was considered. Follow‐up data were complete in all patients. The study was approved by the Great Ormond Street Hospital Ethics committee without requirement of individual consent from patient as the study is based on routinely collected data for clinical purposes as per national recommendations.
Statistical Analysis
Descriptive statistical data are presented as mean (SD) or median (interquartile range) for continuous variables depending on data distribution. Categorical variables are shown as numbers and frequency. Group comparisons are based on parametric tests (unpaired t test) or nonparametric tests (Mann‐Whitney U tests) depending on normality of data distribution. Variables were tested for normality using Q‐Q plots and the Kolmogorov‐Smirnov test. Nonparametric tests were used for nonnormally distributed variables. For categorical variables, group comparisons are based on the χ2 test. To assess the association among demographic, echocardiographic, or hemodynamic parameters, univariate and multivariate Cox proportional hazards regression analyses were performed. Based on the results, survival tree models using recursive partitioning for classification were built using the R package Rpart (R Foundation for Statistical Computing, Vienna, Austria) to produce a simple flowchart‐like risk stratification tool for clinicians. Kaplan‐Meier survival plots were used to illustrate transplantation‐free survival for all terminal nodes in the model. To assess the robustness of the models, the Cox model was applied to 1000 repetitions of a random sample including 80% of the observations. Based on the results, the percentage of samples yielding statistically significant associations with adverse outcome is reported. In addition, we performed a robustness test by randomly replacing 5 observations with a value sampled from the normal distribution of the respective variable in question. This process was also repeated 1000 times, and the percentage of statistically significant associations with adverse outcome on Cox analysis is reported. This test is intended to estimate the impact of natural variation and measurement error on the robustness of the model. Kaplan‐Meier plots are used to illustrate transplant‐free survival by group, and log rank tests were used for group comparison.
For all statistical analyses, R version 3.6.2 was used, and a 2‐sided P value of <0.05 was considered statistically significant.
RESULTS
Overall, 63 patients with PAH were included in the study. Demographic data are presented in Table 1. The mean age at baseline echocardiographic examination was 9.0±5.2 years, and the majority of patients were girls (n=38; 60.3%). Of these, 42 patients (66.7%) had idiopathic pulmonary hypertension, while the remaining patients (n=21; 33.3%) had PAH associated with congenital heart disease or connective tissue disease. At the time of baseline echocardiography 4 patients (6.3%) had no specific PAH therapy, while 24 (38.1%), 19 (30.2%), and 16 (25.4%) were on mono, dual, or triple advanced PAH‐specific therapy, respectively. The most common single‐therapy drug was bosentan (n=12; 19.0%) followed by sildenafil (n=7; 11.1%). Among patients with dual therapy, the combination of bosentan and sildenafil was most commonly used (n=10; 15.9%), followed by the combination of bosentan and epoprostenol (n=6; 9.5%).
Table 1.
Overview of Baseline Characteristics Stratified by Event‐Free Survival
| Demographics and clinical characteristics | Entire cohort | Event‐free patients | Patients with death or transplantation | P value |
|---|---|---|---|---|
| Number of patients | 63 | 42 | 21 | |
| Male sex, n (%) | 25 (39.7) | 16 (38.1) | 9 (42.9) | 0.93 |
| Age at baseline, y, mean (SD) | 9.05 (5.24) | 8.86 (5.35) | 9.44 (5.13) | 0.68 |
| Diagnosis, n (%) | 0.78 | |||
| Associated PHT | 21 (33.3) | 0 (0) | 6 (28.6) | |
| Idiopathic PAH | 42 (66.7) | 27 (100) | 15 (72.5) | |
| Weight, kg, mean (SD) | 28.61 (15.36) | 28.62 (14.35) | 28.60 (17.58) | 1.00 |
| Height, cm, mean (SD) | 125.20 (31.00) | 124.90 (32.19) | 125.85 (29.13) | 0.91 |
| WHO‐FC, n (%) | 0.01 | |||
| I | 6 (10.0) | 5 (12.5) | 1 (5.0) | |
| II | 26 (43.3) | 22 (55.0) | 4 (20) | |
| III | 27 (45) | 13 (32.5) | 14 (70) | |
| IV | 1 (1.7) | 0 (0) | 1 (5.0) | |
| Oxygen saturation in room air, %, median (IQR) | 96.00 (94.00, 98.00) | 97.00 (94.75, 98.00) | 94.00 (92.00, 96.00) | 0.005 |
| 6‐min walk test distance, m, mean (SD) | 352.56 (123.81) | 390.11 (101.55) | 282.47 (134.20) | 0.005 |
| Minimal oxygen saturation at 6‐min walk test, %, median (IQR) | 85.00 (81.50, 91.00) | 86.50 (82.75, 92.25) | 82.00 (71.50, 90.50) | 0.039 |
| Hemodynamic characteristics | ||||
| Mean pulmonary arterial pressure, mm Hg, mean (SD) | 55.8 (24.0) | 48.1 (21.3) | 71.7 (21.8) | 0.001 |
| Mean aortic pressure, mm Hg, mean (SD) | 63.1 (10.3) | 63.4 (8.5) | 62.6 (13.6) | 0.80 |
| Right arterial pressure, mm Hg, median (IQR) | 7.0 (5.0, 8.0) | 6.0 (5.0, 8.0) | 8.0 (7.3, 13.0) | 0.017 |
| Left atrial pressure, mm Hg, median (IQR) | 8.0 (7.0, 10.0) | 8.0 (6.0, 9.0) | 8.5 (7.0, 11.0) | 0.44 |
| Pulmonary artery oxygen saturation, %, median (IQR) | 69.5 (64.3, 75.6) | 73.0 (67.0, 82.0) | 65.0 (57.0, 71.0) | 0.019 |
| PVRI baseline, WU×m2, median (IQR) | 13.6 (9.3, 25.6) | 11.8 (6.5, 22.1) | 24.2 (13.5, 27.4) | 0.01 |
| PVRI with nitric oxide, WU×m2 median (IQR) | 12.0 (5.9, 19.5) | 10.3 (5.0, 15.2) | 15.8 (12.1, 26.2) | 0.03 |
| Echocardiographic characteristics | ||||
| Right heart dimensions | ||||
| Right atrial area, cm2 median (IQR) | 12.83 (9.75, 15.69) | 11.75 (9.28, 13.39) | 15.90 (11.10, 19.20) | 0.008 |
| Right atrial z score, median (IQR) | 2.92 (1.25, 5.11) | 1.77 (1.03, 3.21) | 5.31 (3.30, 7.21) | <0.001 |
| Tricuspid valve diameter, cm, mean (SD) | 2.75 (0.75) | 2.66 (0.68) | 2.95 (0.88) | 0.15 |
| Tricuspid valve diameter, z score, median (IQR) | 2.78 (0.76, 5.23) | 2.04 (0.32, 4.25) | 4.56 (2.31, 5.65) | 0.035 |
| RV internal diastolic diameter, cm, median (IQR) | 2.60 (1.85, 3.53) | 2.44 (1.60, 2.85) | 2.90 (2.28, 4.35) | 0.09 |
| RV internal systolic diameter, cm, median (IQR) | 2.40 (1.76, 3.70) | 2.20 (1.40, 2.60) | 3.45 (2.21, 4.48) | 0.036 |
| Pulmonary valve diameter, cm, mean (SD) | 2.15 (0.61) | 2.07 (0.61) | 2.31 (0.61) | 0.16 |
| Pulmonary valve diameter, z score, mean (SD) | 2.28 (2.66) | 1.72 (2.78) | 3.24 (2.17) | 0.04 |
| Right pulmonary artery dimension, cm mean (SD) | 1.44 (0.51) | 1.39 (0.52) | 1.53 (0.48) | 0.33 |
| Right pulmonary artery dimension, z score, mean (SD) | 1.72 (2.33) | 1.42 (2.47) | 2.24 (2.02) | 0.20 |
| Left pulmonary artery dimension, cm, mean (SD) | 1.45 (1.12, 1.97) | 1.44 (1.10, 1.95) | 1.50 (1.20, 2.00) | 0.56 |
| Left pulmonary artery dimension, z score, mean (SD) | 2.21 (1.02, 4.92) | 2.21 (0.56, 4.56) | 2.21 (1.11, 5.25) | 0.37 |
| Right ventricular function | ||||
| Semiquantitative RV function, I–IV, median (IQR) | 2.00 (1.00, 2.50) | 1.25 (1.00, 2.00) | 2.50 (2.00, 3.00) | <0.001 |
| Tricuspid annular plane systolic excursion, cm, mean (SD) | 1.59 (0.42) | 1.66 (0.45) | 1.47 (0.34) | 0.10 |
| Tricuspid annular plane systolic excursion, z score, mean (SD) | −2.77 (2.40) | −2.36 (2.52) | −3.56 (1.95) | 0.07 |
| RV dp.dt, median (IQR) | 1143 (964, 1620) | 1131 (966, 1403) | 1236 (924, 1629) | 0.75 |
| RV shortening fraction, %, mean (SD) | 39.5 (12.2) | 38.1 (11.7) | 42.3 (13.0) | 0.21 |
| PH severity parameters | ||||
| Tricuspid regurgitation Doppler velocity, m/s, mean (SD) | 4.27 (0.92) | 3.99 (0.85) | 4.79 (0.82) | 0.001 |
| Pulmonary acceleration time, ms, median (IQR) | 67.0 (51.0, 83.0) | 75.8 (59.5, 98.5) | 50.0 (47.0, 73.5) | 0.008 |
| Left heart dimensions | ||||
| Left atrial area, cm2, median (IQR) | 8.08 (3.48) | 8.09 (3.45) | 8.07 (3.62) | 0.98 |
| Left atrial z score, median (IQR) | −1.01 (−1.81, 0.55) | −1.02 (−1.75, 0.35) | −0.89 (−2.00, 0.76) | 0.90 |
| Mitral valve diameter, cm, mean (SD) | 1.98 (0.55) | 2.04 (0.56) | 1.84 (0.51) | 0.19 |
| Mitral valve diameter, z score, median (IQR) | −2.36 (2.46) | −2.18 (2.62) | −2.73 (2.09) | 0.42 |
| LV internal diastolic diameter, cm, median (IQR) | 31.72 (8.86) | 34.07 (8.38) | 27.26 (8.16) | 0.004 |
| LV internal systolic diameter, cm, median (IQR) | 19.24 (6.63) | 20.83 (5.63) | 16.21 (7.45) | 0.01 |
| Aortic valve diameter, cm, mean (SD) | 1.48 (0.38) | 1.47 (0.38) | 1.50 (0.40) | 0.75 |
| Aortic valve diameter, z score, mean (SD) | −1.92 (1.23) | −1.97 (1.33) | −1.81 (1.02) | 0.64 |
| Left ventricular function | ||||
| Ejection fraction, %, mean (SD) | 69.6 (14.8) | 67.8 (14.1) | 73.2 (16.0) | 0.19 |
| Left ventricular eccentricity indices | ||||
| Systolic LV eccentricity index, median, (IQR) | 1.9 (1.5, 2.5) | 1.70 (1.5, 2.2) | 2.4 (1.7, 3.4) | 0.014 |
| Diastolic LV eccentricity index, median, (IQR) | 1.5 (1.3, 1.8) | 1.3 (1.2, 1.6) | 1.8 (1.4, 2.0) | 0.001 |
| Tissue Doppler characteristics | ||||
| Tricuspid valve S‐wave, m/s, mean (SD) | 0.11 (0.02) | 0.11 (0.02) | 0.10 (0.03) | 0.29 |
| Tricuspid valve E‐wave, m/s, mean (SD) | 0.12 (0.04) | 0.13 (0.04) | 0.11 (0.05) | 0.15 |
| Tricuspid valve A‐wave, m/s, mean (SD) | 0.11 (0.03) | 0.11 (0.03) | 0.11 (0.02) | 0.75 |
| Interventricular septum S‐wave, m/s, median (IQR) | 0.06 (0.05, 0.07) | 0.06 (0.05, 0.07) | 0.06 (0.04, 0.07) | 0.12 |
| Interventricular septum E‐wave, m/s, median (IQR) | 0.07 (0.06, 0.10) | 0.08 (0.06, 0.10) | 0.06 (0.04, 0.08) | 0.02 |
| Interventricular septum A‐wave, m/s, median (IQR) | 0.06 (0.05, 0.07) | 0.06 (0.05, 0.06) | 0.06 (0.05, 0.07) | 0.70 |
| Mitral valve S‐wave, m/s, mean (SD) | 0.06 (0.05, 0.08) | 0.06 (0.06, 0.08) | 0.06 (0.05, 0.07) | 0.06 |
| Mitral valve E‐wave, m/s, mean (SD) | 0.11 (0.04) | 0.12 (0.04) | 0.09 (0.05) | 0.01 |
| Mitral valve A‐wave, m/s, mean (SD) | 0.05 (0.05, 0.07) | 0.05 (0.05, 0.06) | 0.06 (0.04, 0.08) | 0.34 |
P values refer to comparisons between patients with and without transplant‐free survival. dp/dt indicates delta pressure/delta time; IQR, interquartile range; IVS, interventricular septum; LV, left ventricular; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PVRI, pulmonary vascular resistance index; RV, right ventricular; WHO‐FC, World Health Organization functional classification; and WU, Wood units.
During a median follow‐up time of 4.0 (interquartile range, 1.3–7.3) years, 17 patients died, and 4 patients underwent a lung transplantation. Transplant‐free survival at 1, 3, and 5 years of follow‐up was 85.4% (95% CI, 77.0%–94.7%), 74.4% (95% CI, 64.0%–86.6%), and 66.2% (95% CI, 54.8%–80.1%), respectively.
Prognostic Value of Demographic Variables and Nonechocardiographic Parameters
Age (hazard ratio [HR], 1.06; 95% CI, 0.97–1.16; P=0.22), sex (P=0.96), and underlying diagnosis (associated PAH versus idiopathic PAH; P=0.71) were not significantly related to transplant‐free survival on univariate Cox analysis. Patients in World Health Organization class ≥3 had a significantly increased risk of transplantation or death (HR, 3.47; 95% CI, 1.26–9.58; P=0.016) compared with patients in World Health Organization class 1 or 2. Additional nonechocardiographic parameters significantly related to transplant‐free survival were oxygen saturation at rest (HR per %, 0.836; 95% CI, 0.751–0.929; P<0.001), 6‐minute walk test distance (HR per m, 0.995; 95% CI, 0.992–0.999; P=0.02), and minimal oxygen saturation at 6‐minute walk test (HR per %, 0.932; 95% CI, 0.893–0.972; P=0.001). In contrast, systolic blood pressure was not significantly related to the combined end point (P=0.23). Among the invasive hemodynamic parameters, mean pulmonary arterial pressure (HR per 10 mm Hg, 1.21; 95% CI, 1.05–1.40; P=0.01), right atrial (RA) pressure (HR per mm Hg, 1.137; 95% CI, 1.009–1.280; P=0.035), and pulmonary artery saturations (HR per %, 0.940; 95% CI, 0.897–0.984; P=0.008) were significantly associated with worse outcome, while aortic pressure (P=0.64), left atrial pressure (P=0.21), and pulmonary vascular resistance index at baseline (P=0.14), pulmonary vascular resistance index with oxygen (P=0.89) and pulmonary vascular resistance index with nitric oxide (P=0.17) were not significantly related to outcome on univariate Cox analysis.
Prognostic Value of Echocardiographic Parameters
As illustrated in Table 2, numerous echocardiographic variables were significantly associated with transplant‐free survival on univariate Cox proportional hazard analysis. Significant predictors of outcome related to the left heart included tissue Doppler parameters of left ventricular (LV) filling (E and A wave at the lateral mitral valve annulus), LV diameters, and LV eccentricity index (Figure 1). For the right heart, right ventricular (RV) dimensions and semiquantitative RV function as well as the dimensions of the tricuspid and pulmonary valve were significantly correlated with outcome. Furthermore, the severity of PAH as indicated by tricuspid regurgitation (TR) velocity and pulmonary acceleration time were predictive on univariate Cox analysis. RA area also emerged as an important prognostic parameter. Interestingly, tricuspid annular plane systolic excursion (TAPSE) was not significantly related to transplant‐free survival, neither in absolute terms (P=0.30) nor after adjustment for patients’ body size (TAPSE z score, P=0.12).
Table 2.
Univariate Predictors of Transplant‐Free Survival
| Parameter | n | HR | 95% CI | P value | c‐value |
|---|---|---|---|---|---|
| Significant parameters | |||||
| E‐wave lateral MV, mm/s | 50 | 0.83 | 0.722–0.955 | 0.009 | 0.71* |
| A‐wave lateral MV, mm/s | 50 | 1.281 | 1.025–1.602 | 0.030 | 0.60 |
| RV semiquant function (>2) | 63 | 3.571 | 1.508–8.456 | 0.000 | 0.66* |
| TV velocity, m/s | 56 | 1.869 | 1.154–3.027 | 0.011 | 0.65* |
| TV diameter, cm | 62 | 1.897 | 1.046–3.438 | 0.035 | 0.62 |
| TV diameter, z score | 62 | 1.129 | 1.008–1.265 | 0.036 | 0.66* |
| RA area, cm2 | 63 | 1.092 | 1.048–1.138 | 0.000 | 0.69 |
| RA area, z score | 63 | 1.11 | 1.057–1.164 | 0.000 | 0.78* |
| Pulmonary valve diameter, z score | 55 | 1.169 | 1.01–1.354 | 0.037 | 0.67* |
| RV internal diameter diastolic, cm | 43 | 1.607 | 1.089–2.371 | 0.017 | 0.61 |
| RV internal diameter systolic, cm | 43 | 1.063 | 1.011–1.118 | 0.016 | 0.66* |
| PA acceleration time, ms | 41 | 0.972 | 0.946–0.999 | 0.042 | 0.69 |
| LV eccentricity index systolic | 60 | 1.908 | 1.219–2.987 | 0.005 | 0.65 |
| LV eccentricity index diastolic | 59 | 7.251 | 2.936–17.908 | 0.000 | 0.72* |
| LV internal diameter systolic, cm | 58 | 0.9407 | 0.987–0.987 | 0.012 | 0.67 |
| LV internal diameter diastolic, cm | 58 | 0.913 | 0.85–0.981 | 0.013 | 0.68* |
| Parameters with P values between 0.05 and 0.10 | |||||
| E‐wave IVS, mm/s | 51 | 0.846 | 0.71–1.007 | 0.060 | 0.69 |
| Pulmonary valve diameter, cm | 55 | 2.11 | 0.948–4.693 | 0.067 | 0.63 |
| Right PA, z score | 57 | 1.183 | 0.985–1.42 | 0.072 | 0.63 |
| Left PA, z score | 58 | 1.177 | 0.982–1.141 | 0.078 | 0.57 |
| LV ejection function, % | 58 | 1.028 | 0.995–1.061 | 0.093 | 0.59 |
HR indicates hazard ratio; IVS, interventricular septum; LV, left ventricular; MV, mitral valve; PA, pulmonary artery; RA, right atrial; RV, right ventricular; and TV, tricuspid valve.
Variables included in the bivariate analyses.
Figure 1. Example of an echocardiographic image of a parasternal short‐axis view.
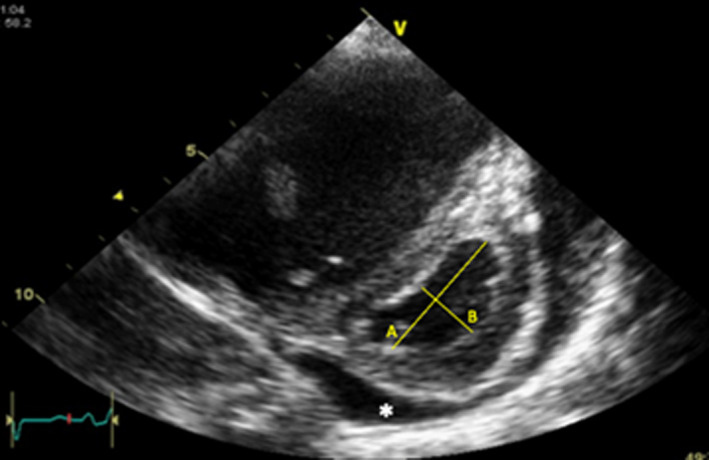
The figure illustrates how left ventricular eccentricity index (LVEI) can be derived from 2‐dimensional imaging. LVEI is expressed as the ratio of A/B. Ratio can be measured in systole or diastole. In addition, there is a small amount of pericardial effusion (*) visible around the left ventricle.
Based on the results of the univariate Cox analysis, bivariate analyses including the parameter with the highest c‐value on univariate testing (ie, RA area z score, c=0.78) were performed. The results of the bivariate analysis are presented in Table 3. Among the parameters tested, TR velocity, systolic RV inflow diameter, LV diastolic eccentricity index, and LV diastolic inflow diameter were significantly related to outcome independently of RA area z score. Including all significant parameters on bivariate analysis in a multivariate Cox model revealed that only RA z score area (HR per unit, 1.107; 95% CI, 1.008–1.216; P=0.03) remained significantly associated with transplant‐free survival.
Table 3.
Bivariate Predictors of Transplant‐Free Survival
| Parameter | n | HR | 95% CI | P value | c‐value |
|---|---|---|---|---|---|
| RA area, z score | 50 | 1.238 | 1.099–1.395 | <0.001 | 0.83 |
| E‐wave lateral MV, mm/s | 0.865 | 0.745–1.005 | 0.06 | ||
| RA area, z score | 63 | 1.090 | 1.035–1.147 | 0.001 | 0.77 |
| RV semiquant function (>2) | 2.520 | 0.993–6.395 | 0.052 | ||
| RA area, z score | 63 | 1.098 | 1.045–1.154 | <0.001 | 0.74 |
| TV velocity, m/s | 1.698 | 1.031–2.795 | 0.037 | ||
| RA area, z score | 63 | 1.120 | 1.037–1.210 | 0.004 | 0.78 |
| TV diameter, z score | 0.975 | 0.843–1.128 | 0.74 | ||
| RA area, z score | 55 | 1.092 | 1.035–1.151 | 0.001 | 0.75 |
| Pulmonary valve diameter, z score | 1.091 | 0.920–1.295 | 0.32 | ||
| RA area, z score | 43 | 1.098 | 1.044–1.156 | <0.001 | 0.76 |
| RV internal diameter systolic, cm | 1.067 | 1.010–1.126 | 0.02 | ||
| RA area, z score | 41 | 1.236 | 1.080–1.414 | 0.002 | 0.82 |
| PA acceleration time, ms | 0.982 | 0.954–1.010 | 0.21 | ||
| RA area, z score | 59 | 1.063 | 1.008–1.120 | 0.020 | 0.75 |
| LV eccentricity index diastolic | 5.508 | 1.926–15.750 | 0.001 | ||
| RA area, z score | 58 | 1.109 | 1.056–1.165 | <0.001 | 0.80 |
| LV internal diameter diastolic, cm | 0.938 | 0.892–0.987 | 0.01 |
HR indicates hazard ratio; LV, left ventricular; MV, mitral valve; RA, right atrial; RV, right ventricular; and TV, tricuspid valve.
Robustness Check of Significant Echocardiographic Parameters
Before building the definitive prognostic model based on the results of the Cox proportional hazard analysis, the robustness of the Cox results to observation selection and potential measurement error was assessed. To this end, all significant parameters on univariate Cox analysis were subjected to a random sampling process where 80% of the observations were selected at random (without replacement), and the significant association of this subsample on univariate Cox analysis with transplant‐free survival was assessed. Table S1 shows the results of the analysis, revealing that only RA area, LV eccentricity index, and semiquantitative RV systolic function remained statistically significant in >95% of cases (ie, >950/1000), while some parameters remained significant in less than half of cases, suggesting a lack of robustness to patient exclusion. Similarly, when replacing 5 randomly selected observations with a random value from the normal distribution of the respective variable, RA area, LV eccentricity index and semiquantitative RV systolic function emerged as most robust to this simulation of possible measurement error (Table S1).
Prognostic Scores and Regression Tree
To enable clinicians to apply the results of the current study to their local patient cohort and to enable external validation of the results, we provide a simplified risk stratification tool. Based on the results of the univariate and bivariate Cox proportional hazard analysis we developed a score system based on the parameters with the highest c‐statistic scores, RA area z score and LV diastolic eccentricity index. Both parameters were dichotomized at their rounded median value (3 for RA area z score and 1.5 for LV diastolic eccentricity index). One point was assigned for each parameter above the median, yielding a score from 0 to 2 (0: RA area z score <3 and LV diastolic eccentricity index <1.5; 1: either RA area z score ≥3 or LV diastolic eccentricity index ≥1.5; 2: both RA area z score ≥3 and LV diastolic eccentricity index ≥1.5). Figure 2 illustrates the Kaplan‐Meier plot for the 3 groups.
Figure 2. Echocardiographic pronostic factors for transplant‐free survival.
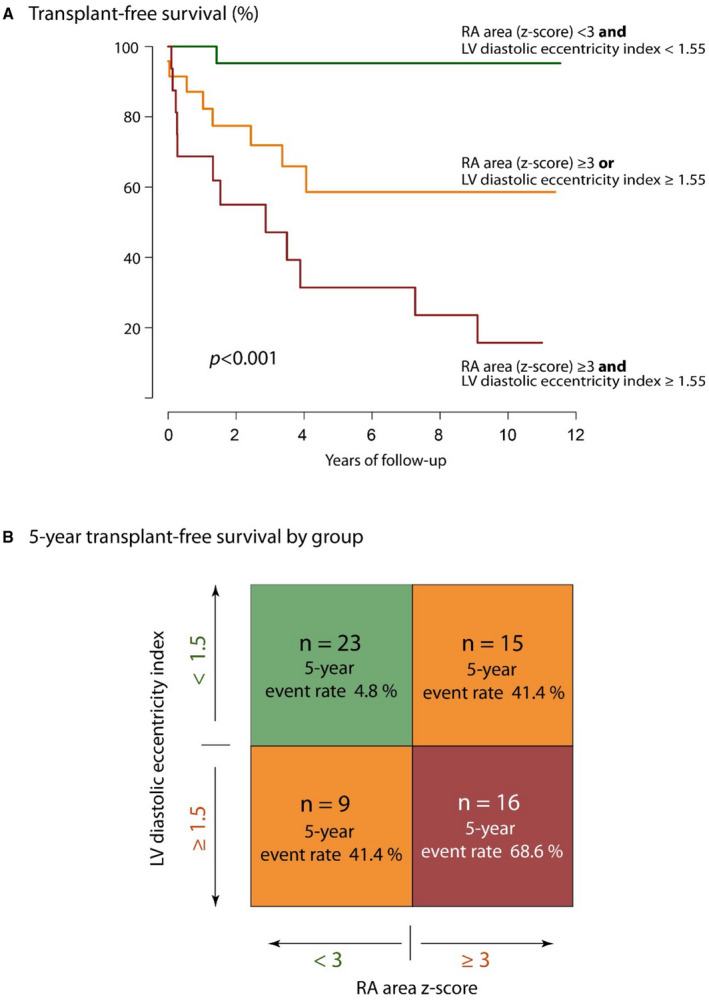
A, Transplant‐free survival of patients according to the risk score based on median values for right atrial (RA) size z score and left ventricular (LV) eccentricity index. The P value refers to the results of the log rank analysis. B, Overview over the distribution of patients to different score categories (according to risk factors) and 5‐year transplant‐free survival by group.
In addition, a survival tree model using recursive partitioning for classification was used to produce a simple flowchart‐like risk stratification tool. Unlike in the above analysis, the algorithm automatically chooses the most appropriate cutoff value for splitting continuous variables, and more complex models are possible. The results of the recursive partitioning process were surprisingly similar to the manual process described above, resulting in a combination of RA area z score and LV diastolic eccentricity index with automatically selected cutoff points (3 and 5.7 for the former, and 1.55 for the latter, respectively). The respective flowchart with corresponding Kaplan‐Meier transplant‐free survival curves by group are presented in Figure 3.
Figure 3. Risk stratification based on the results of the survival tree models using recursive partitioning for classification.
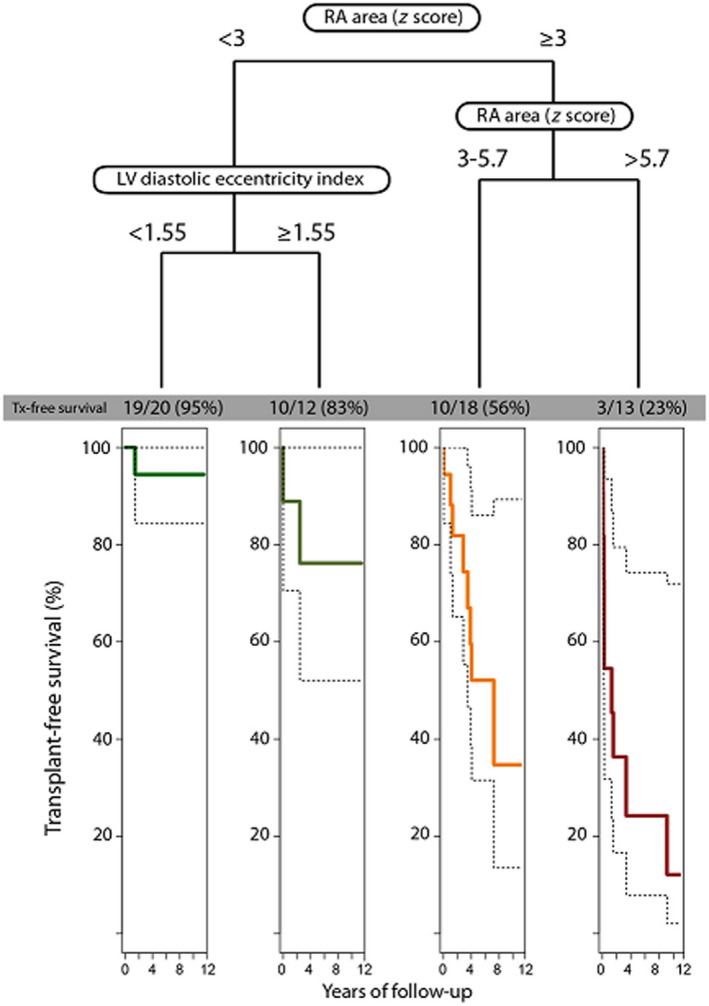
Patients are stratified into 4 risk groups based on baseline right atrial (RA) area z score and left ventricular (LV) diastolic eccentricity index. The Kaplan‐Meier curves illustrate the transplant‐free survival prospects of each group, respectively. Tx indicates transplant.
The association between echocardiographic parameters and mortality alone was assessed as a secondary end point. During a median follow‐up time of 4.1 years, 17 patients died. Patients who underwent lung transplantation were censored at the time of transplantation for this subanalysis. As illustrated in Table S2, various echocardiographic parameters were significantly associated with the risk of death on univariate Cox proportional hazard analysis. On bivariate and multivariate analysis, RA area z score, LV eccentricity index, and TAPSE z score emerged as significant predictors and were incorporated in the final prognostic model (Tables 4 and 5 and Figure 4).
Table 4.
Predictors of Mortality on Univariate Cox Proportional Hazard Analysis
| Parameter | n | HR | 95% CI | P value | c‐value |
|---|---|---|---|---|---|
| A‐wave lateral MV, mm/s | 50 | 1.389 | 1.099–1.755 | 0.006 | 0.67 |
| RV semiquantitaive function (>1) | 63 | 8.147 | 1.072–61.91 | 0.043 | 0.65 |
| TV diameter, cm | 62 | 1.989 | 1.022–3.871 | 0.043 | 0.61 |
| TV diameter, z score | 62 | 1.216 | 1.082–1.367 | 0.001 | 0.71 |
| RA area, cm2 | 63 | 1.103 | 1.053–1.155 | <0.001 | 0.68 |
| RA area, z score | 63 | 1.121 | 1.06–1.185 | <0.001 | 0.75 |
| Right pulmonary artery, cm | 57 | 2.796 | 1.042–7.503 | 0.041 | 0.59 |
| Right pulmonary artery, z score | 57 | 1.297 | 1.054–1.597 | 0.142 | 0.67 |
| Left pulmonary artery, z score | 58 | 1.26 | 1.033–1.537 | 0.023 | 0.60 |
| LV eccentricity index systolic | 60 | 1.779 | 1.095–2.89 | 0.020 | 0.64 |
| LV eccentricity index diastolic | 59 | 6.301 | 2.74–14.49 | <0.001 | 0.76 |
| TAPSE, z score | 58 | 0.7558 | 0.591–0.971 | 0.028 | 0.64 |
LV indicates left ventricular; MV, mitral valve; RA, right atrial; RV, right ventricular; and TAPSE, tricuspid annular plain systolic excursion.
Table 5.
Bivariate and Multivariate Analysis of Predictors of Mortality Based on the Results of the Univariate Cox Proportional Hazard Analysis (See Table 4)
| Parameter | n | HR | 95% CI | P value | c‐value |
|---|---|---|---|---|---|
| Bivariate analyses | |||||
| LV eccentricity index diastolic† | 46† | 3.216† | 1.123–9.21† | 0.030† | 0.77† |
| A‐wave lateral MV, mm/s | 1.211 | 0.931–1.576 | 0.15 | 0.67 | |
| LV eccentricity index diastolic† | 46† | 5.021† | 2.067–12.2† | <0.001† | 0.78† |
| RV semiquant function (>1) | 3.872 | 0.482–31.12 | 0.20 | ||
| LV eccentricity index diastolic† | 58† | 4.537† | 1.749–11.773† | 0.002† | 0.76† |
| TV diameter (z score) | 1.12 | 0.989–1.268 | 0.07 | ||
| LV eccentricity index diastolic† | 58† | 4.804† | 1.851–12.47† | 0.001† | 0.78† |
| RA area, z score† | 1.074† | 1.013–1.14† | 0.02† | ||
| LV eccentricity index diastolic† | 53† | 4.402† | 1.739–11.144† | 0.002† | 0.77† |
| Right pulmonary artery, z score | 1.154 | 0.914–1.456 | 0.23 | ||
| LV eccentricity index diastolic† | 55† | 5.649† | 2.274–14.03† | <0.001† | 0.79† |
| TAPSE, z score† | 0.764† | 0.596–0.98† | 0.03† | ||
| Multivariate analyses | |||||
| LV eccentricity index diastolic† | 55† | 4.078† | 1.388–11.986† | 0.01† | 0.82† |
| RA area, z score† | 1.085† | 1.2–1.154† | 0.01† | ||
| TAPSE, z score† | 0.746† | 0.578–0.963† | 0.02† | ||
LV indicates left ventricular; MV, mitral valve; RA, right atrial; RV, right ventricular; and TAPSE, tricuspid annular plain systolic excursion.
Variables included in the bivariate analyses.
Figure 4. Outcome according to combined echocardiographic score.
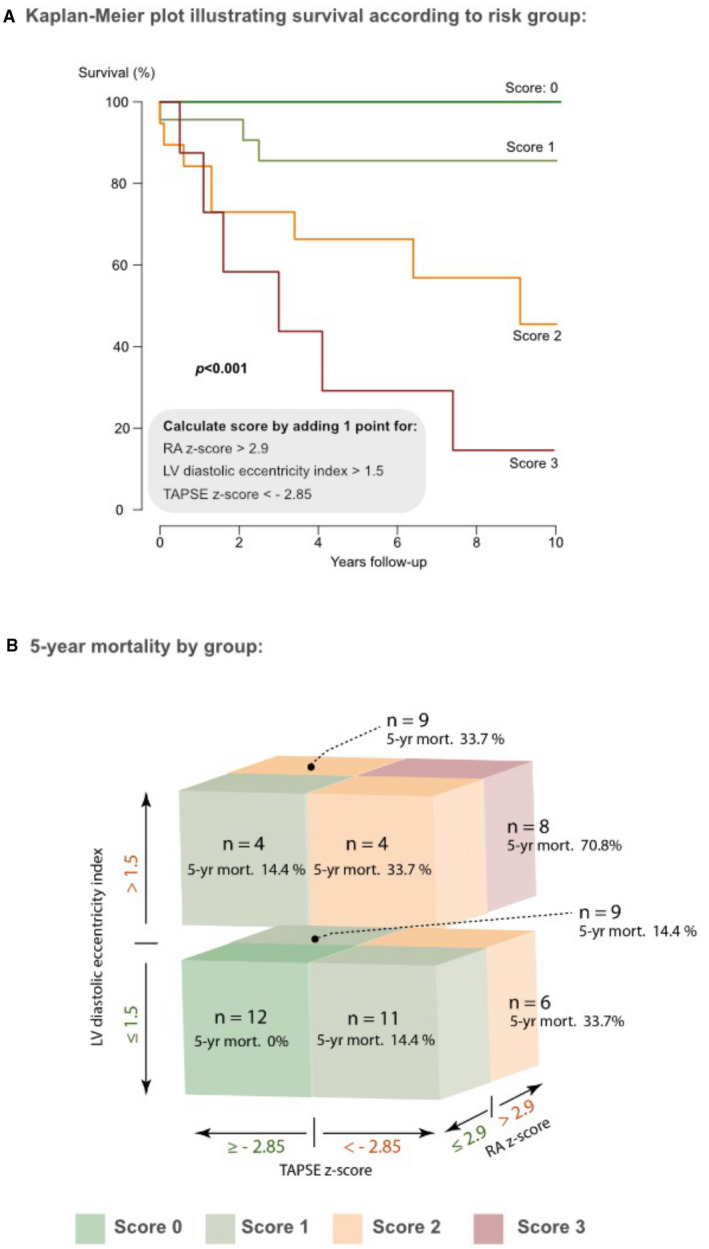
A, Survival prospects of patients according to the additive risk score based on median values for right atrial (RA) size z score, left ventricular (LV) eccentricity index, and tricuspid annular plane systolic excursion (TAPSE) z score. B, Overview over the distribution of patients to different score groups (according to their risk factors) and respective 5‐year survival by group.
DISCUSSION
PH is associated with high morbidity and mortality in pediatric patients. 1 , 2 , 17 Based on data from a large national referral center for pediatric PAH, we report the prognostic value of routine and tissue Doppler echocardiographic parameters and propose a simple risk stratification score that should be widely applicable. We propose the combination of RA size and LV eccentricity index as a powerful prognostic marker of transplant‐free survival, potentially to be complemented by TAPSE for predicting mortality alone.
Previous studies have assessed the prognostic value of echocardiographic parameters in children with PH. Beyond including a larger number of patients, our study complements and extends these results by a larger number of events and by proposing unified risk models. Kassem and colleagues studied 54 children with PH. 14 During a median follow‐up time of 2.5 years for patients with idiopathic PAH and 4.3 years for patients with associated PAH, 9 events of death or transplantation occurred. The authors report that RV fractional area change, TAPSE z score, RV end‐diastolic dimensions (z score) and RV end‐systolic area index were predictive of outcome in isolation. More recently, Ploegstra et al 15 reported their experience on the basis of 43 children with PH. Over a median period of 5.8 years, there were 15 deaths and 3 lung transplantations. LV dimensions, RV/LV ratio, RV ejection time, and nonindexed TAPSE predicted outcome on univariate Cox analysis. Consistent with previous studies, TAPSE also emerged as a significant predictor of death in our study. 18 Perhaps surprisingly, however, we found RA area and LV eccentricity index to have superior discriminative ability and to be more robust compared with TAPSE, especially for the combined end point, even when TAPSE was adjusted for children’s age in our cohort. When mortality alone was considered as an end point, however, TAPSE emerged as an independent predictor of outcome and should thus be considered as an adjunct in the risk stratification process. Our analysis emphasizes the potential importance and robustness of RA area size and LV eccentricity index for prognostication in children with PAH (Figure 4). Using a combination of these parameters, a flowchart‐like risk prediction model was constructed, differentiating patients well by prognosis (Figure 4). We accept that this model awaits external validation but contend that its simplicity should facilitate this essential step. In addition, it can be calculated at the bedside without computer‐assisted tools.
The results of our study highlight that PAH exerts a multiplicity of effects on the heart unit, which in turn informs prognosis.
The data‐driven combination of independently prognostic parameters appears intriguing, as it provides information of different physiological consequences of PAH. RA dilatation has repeatedly been demonstrated to be prognostic in pediatric 19 and adult imaging studies. Similarly, RA pressure has been established as a robust prognostic marker in this setting. 20 , 21 RA distention may result from RV diastolic or systolic dysfunction, tricuspid valve regurgitation, and fluid overload. As such, it may represent a composite biomarker of processes known to negatively impact outcome. The LV eccentricity index is a quantitative description of the extent of interventricular septal deviation. In contrast to the systolic LV eccentricity index—related to the relative pressure difference between RV and LV—the diastolic LV eccentricity index emerged as a superior prognostic marker in the current study. Diastolic LV eccentricity index is an electromechanical phenomenon that is the result of prolonged RV contraction in response to elevated pulmonary artery pressure (Figure 5). 22 As such, it is linked to afterload. Furthermore, in severe PAH, RV systole continues into LV diastole. This phenomenon contributes to ventricular‐ventricular interaction. The importance of the diastolic (or maximal) LV eccentricity index for identifying severe PAH and adverse outcome has also been recently highlighted by Burkett et al. 23
Figure 5. Prolonged RV contraction, which is a response to elevated pulmonary artery pressure.
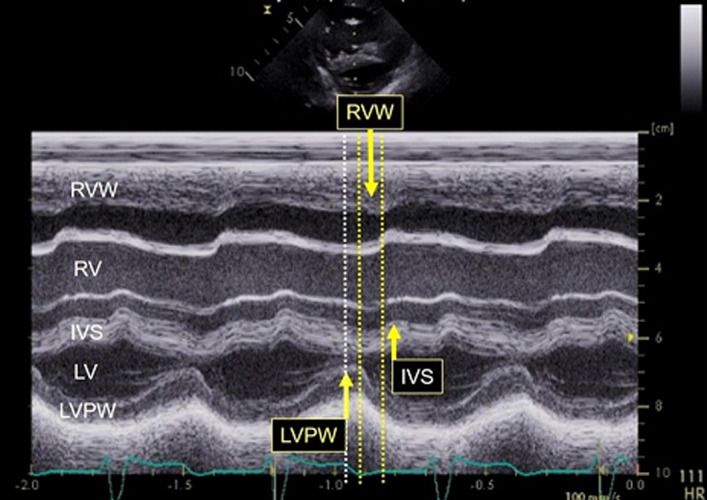
IVS indicates interventricular septum; LV, left ventricule; LVPW, left ventricular posterior wall; RV, right ventricule; and RVW right ventricular (anterior) wall.
Furthermore, it is the main mechanism of electromechanical ventriculo‐ventricular interaction. The greater the deviation—particularly in diastole—the more restriction in LV filling, and hence cardiac output, is expected. This abnormal interaction reflects delayed mechanical septal contraction and the right bundle branch block where the septum continues to contract while the LV begins to fill.
Finally, TAPSE is a simple and relatively robust measure of RV (longitudinal) function, which itself is an independent mechanistic prognostic marker. 18 Thus, the 3 components of our proposed risk score capture the important physiological perturbations resulting from PH.
Limitations
Patients included in the current study were under regular follow‐up and treatment. Physicians were not blinded to the echocardiographic findings when making therapeutic decisions. Strain measurements were not part of the current analysis, and further studies are required to clarify the potential prognostic value of these parameters. Because of the limited number of events, we chose to focus on an echocardiographic risk stratification score and refrained from incorporating hemodynamic or functional parameters directly into the model (many of them not performed at the exact same time as echocardiographic assessment). We accept that TAPSE is load dependent and may be affected by significant TR in the same way as RA enlargement may be related to tricuspid valve dysfunction. In our study, however, only 1 patient had severe TR, and the severity of TR was not statistically correlated with RA area or TAPSE (P values of 0.32 and 0.13, respectively). The current analysis is focused on echocardiographic markers. Invasive hemodynamic parameters were thus not included in the prediction algorithm. While some invasive parameters (eg, mean pulmonary arterial pressure, RA pressure, and pulmonary artery saturations) were significantly related to prognosis, others—most notably pulmonary vascular resistance index—were not. This finding requires further study as part of protocols focusing specifically on invasive hemodynamic parameters. In addition, further multicenter studies with a larger number of events are required to produce a comprehensive pediatric PH risk score incorporating broader demographic, clinical, functional, hemodynamic, and imaging parameters.
Conclusions
Echocardiographic parameters predict prognosis in children with PAH. A combination of widely available parameters including RA area, LV eccentricity index, and TAPSE emerged as a simple risk stratification score that may assist clinicians to determine the prognosis of pediatric patients with PAH.
Sources of Funding
The authors have no external funding to declare.
Disclosures
None.
Supporting information
Tables S1–S2
Acknowledgments
Sheila G. Haworth is deceased. It is with sadness that we inform the reader that Professor Haworth has passed away. Her dedication and contribution were central in this work and beyond. We sincerely thank all clinicians associated with the UK Pulmonary Hypertension Service for Children, who share the care for these complex patients. Without their cooperation and collaborative work, this study would not have been possible. All research at Great Ormond Street Hospital National Health System Foundation Trust and University College London Great Ormond Street Institute of Child Health is made possible by the National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health System, the National Institute for Health Research, or the Department of Health.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023118
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Alonso‐Gonzalez R, Lopez‐Guarch CJ, Subirana‐Domenech MT, Ruíz JMO, González IO, Cubero JS, del Cerro MJ, Salvador ML, Subira LD, Gallego P, et al. Pulmonary hypertension and congenital heart disease: an insight from the REHAP National Registry. Int J Cardiol. 2015;184:717–723. doi: 10.1016/j.ijcard.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 2. Ivy DD, Abman SH, Barst RJ, Berger RMF, Bonnet D, Fleming TR, Haworth SG, Raj JU, Rosenzweig EB, Schulze Neick I, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62:D117–D126. doi: 10.1016/j.jacc.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 3. Barst R. How has epoprostenol changed the outcome for patients with pulmonary arterial hypertension? Int J Clin Pract Suppl. 2010;64:23–32. doi: 10.1111/j.1742-1241.2010.02525.x [DOI] [PubMed] [Google Scholar]
- 4. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani H‐A, Jansa P, Jing Z‐C, Le Brun F‐O, Mehta S, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 5. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani H‐A, Hoeper MM, Lang IM, Preiss R, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 6. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 7. Lammers AE, Burch M, Benden C, Elliott MJ, Rees P, Haworth SG, Aurora P. Lung transplantation in children with idiopathic pulmonary arterial hypertension. Pediatr Pulmonol. 2010;45:263–269. doi: 10.1002/ppul.21168 [DOI] [PubMed] [Google Scholar]
- 8. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society , et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 9. Haarman MG, Douwes JM, Ploegstra MJ, Roofthooft MTR, Vissia‐Kazemier TR, Hillege HL, Berger RMF. The clinical value of proposed risk stratification tools in pediatric pulmonary arterial hypertension. Am J Respir Crit Care Med. 2019;200:1312–1315. doi: 10.1164/rccm.201902-0266LE [DOI] [PubMed] [Google Scholar]
- 10. Lammers AE. Predicting prognosis of children with pulmonary arterial hypertension: the importance of multimodal expert assessment. Heart. 2014;100:1305–1307. doi: 10.1136/heartjnl-2014-306010 [DOI] [PubMed] [Google Scholar]
- 11. Lammers AE, Haworth SG, Riley G, Maslin K, Diller GP, Marek J. Value of tissue Doppler echocardiography in children with pulmonary hypertension. J Am Soc Echocardiogr. 2012;25:504–510. doi: 10.1016/j.echo.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 12. Lammers AE, Hislop AA, Haworth SG. Prognostic value of B‐type natriuretic peptide in children with pulmonary hypertension. Int J Cardiol. 2009;135:21–26. doi: 10.1016/j.ijcard.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 13. Douwes JM, Hegeman AK, van der Krieke MB, Roofthooft MT, Hillege HL, Berger RM. Six‐minute walking distance and decrease in oxygen saturation during the six‐minute walk test in pediatric pulmonary arterial hypertension. Int J Cardiol. 2016;202:34–39. doi: 10.1016/j.ijcard.2015.08.155 [DOI] [PubMed] [Google Scholar]
- 14. Kassem E, Humpl T, Friedberg MK. Prognostic significance of 2‐dimensional, M‐mode, and Doppler echo indices of right ventricular function in children with pulmonary arterial hypertension. Am Heart J. 2013;165:1024–1031. doi: 10.1016/j.ahj.2013.02.027 [DOI] [PubMed] [Google Scholar]
- 15. Ploegstra MJ, Roofthooft MT, Douwes JM, Bartelds B, Elzenga NJ, van de Weerd D, Hillege HL, Berger RM. Echocardiography in pediatric pulmonary arterial hypertension: early study on assessing disease severity and predicting outcome. Circ Cardiovasc Imaging. 2015;8. doi: 10.1161/CIRCIMAGING.113.000878 [DOI] [PubMed] [Google Scholar]
- 16. Okumura K, Humpl T, Dragulescu A, Mertens L, Friedberg MK. Longitudinal assessment of right ventricular myocardial strain in relation to transplant‐free survival in children with idiopathic pulmonary hypertension. J Am Soc Echocardiogr. 2014;27:1344–1351. doi: 10.1016/j.echo.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Rich S, Haworth SG, Hassoun PM, Yacoub MH. Pulmonary hypertension: the unaddressed global health burden. Lancet Respir Med. 2018;6:577–579. doi: 10.1016/S2213-2600(18)30268-6 [DOI] [PubMed] [Google Scholar]
- 18. Forfia PR, Fisher MR, Mathai SC, Housten‐Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC [DOI] [PubMed] [Google Scholar]
- 19. Moledina S, Pandya B, Bartsota M, Mortensen KH, McMillan M, Quyam S, Taylor AM, Haworth SG, Schulze‐Neick I, Muthurangu V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:407–414. doi: 10.1161/CIRCIMAGING.112.000082 [DOI] [PubMed] [Google Scholar]
- 20. Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long‐Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122 [DOI] [PubMed] [Google Scholar]
- 21. del Cerro Marin MJ, Sabate Rotes A, Rodriguez Ogando A, Mendoza Soto A, Quero Jimenez M, Gavilan Camacho JL, Raposo Sonnenfeld I, Moya Bonora A, Albert Brotons DC, Moreno Galdo A, et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med. 2014;190:1421–1429. doi: 10.1164/rccm.201406-1052OC [DOI] [PubMed] [Google Scholar]
- 22. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burkett A, Patel SS, Mertens L, Friedberg MK, Dunbar ID. Relationship between left ventricular geometry and invasive hemodynamics in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2020;13:e009825. doi: 10.1161/CIRCIMAGING.119.009825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


