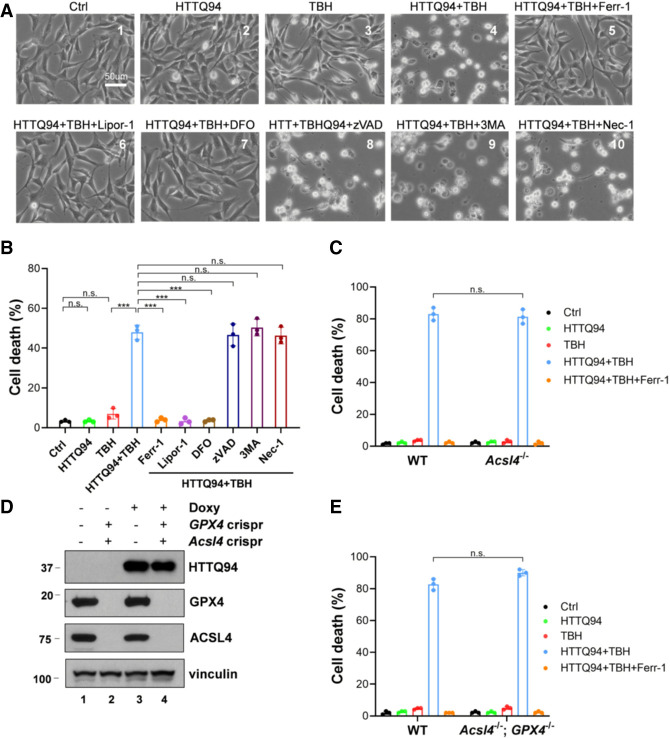
Figure 3.
HTTQ94 is able to induce the ACSL4-independent ferroptosis upon ROS stress. (A) Representative phase-contrast images of cell death from the HTTQ94 tet-on HT-22 cells. HTTQ94 tet-on HT-22 cells were preincubated with 0.5 μg/mL doxycycline for 4 h and then treated with 10 μM TBH for 8 h in the presence or absence of the ferroptosis inhibitors (2 μM ferrostatin-1 [Ferr1], 2 μM liproxstatin-1 [Lipor1], and 100 μM DFO), apoptosis inhibitor (10 μM Z-VADFMK [zVAD]), autophagy inhibitor (2 mM 3-methylademine [3MA]), or necroptosis inhibitor (10 μM necrostatin-1 [Nec1]). (B) Quantification of cell death, related to A. Three replicates were used for each group. (C) HTTQ94 tet-on SK-N-BE(2)C control Crispr and Acsl4Crispr cells were preincubated with 0.5 μg/mL doxycycline for 16 h and then treated with 350 μM TBH for 24 h with/without 2 μM Ferr-1. (D) Western blot analysis of GPX4, ACSL4, and HTTQ94 in HTTQ94 tet-on SK-N-BE(2)C control Crispr and GPX4/Acsl4 double-Crispr cells treated with 0.5 μg/mL doxycycline for 16 h. (E) HTTQ94 tet-on SK-N-BE(2)C control Crispr and GPX4/Acsl4 double-Crispr cells were preincubated with 0.5 μg/mL doxycycline for 16 h and then treated with 350 μM TBH for 24 h with/without 2 μM Ferr-1. Cell deaths were calculated from three replicates; Data shown in B, C, and E are means ± SD. P-values were derived from two-tailed unpaired t-test. (***) P ≤ 0.001, (n.s.) P > 0.05.