Abstract
Objectives
The distinctive bilateral carotid sheaths (CS) reside in the neck region and form part of the deep cervical fasciae. Aspects of the CS anatomy are controversial, most notably its specific attachment sites and fascial makeup, which are key determinants for the spread of tumours and infections and surgical planning. This review aimed to organise the pertinent aspects relating to CS anatomy and pathology, explore their clinical relevance and highlight areas of disagreement in the literature.
Methods
A narrative review identified key papers relating to CS anatomy, histology, embryology, pathology and clinical and surgical significance using PubMed and Google Scholar. This was supported by a systematic review focused on the fascia forming the CS which was conducted using PubMed, Web of Science and Core Collection which yielded 22 papers.
Results
and Discussion: The CS surrounds the internal carotid artery, internal jugular vein, cranial nerves IX - XII, lymph nodes and nervous plexuses as they course from the jugular foramen superiorly down along into the mediastinum inferiorly. There are contradicting descriptions regarding the CS attachments at the extracranial skull base and within the mediastinum. Author descriptions of the CS fasciae are complex, varied and incongruent. Pathologies affecting the CS include malignancies of the nerves, vascular lesions and utilisation of the CS space as a corridor for the spread of deep neck infections.
Conclusion
This paper collates and presents pertinent anatomical and clinical aspects regarding the CS. A proper knowledge of the CS anatomy and structural relationships will optimise surgical approaches and orientation when operating within the region.
Keywords: Carotid sheath, Deep cervical fascia, Internal carotid artery, Internal jugular vein, Lower cranial nerves, Pathology, Surgery
1. Introduction
The carotid sheath (CS) is a fibrous tunnel in the cervical deep fascia that serves to transport the internal carotid artery, internal carotid artery and the lower cranial nerves (CNs IX, X, XI and XII) as they exit the skull base and enter the neck and then the mediastinum. There are a number of descriptions regarding the complex aspects of CS anatomy. These range from the debated fascial contributions which form the sheath, spatial positioning of the sheath's contents, anatomical divisions of the sheath, relationships with other fascia and neck compartments, proposed models of carotid sheath arrangement, and contradictions regarding the superior and inferior sheath attachment sites. Some authors have even argued against the existence of a discrete sheath structure in the neck. A few papers have also investigated histology and microstructure of the CS, and there are several descriptions of CS embryology, its mesodermal origin, and its formation after development of the vessels. Awareness of carotid sheath anatomy is very pertinent in the clinical context. There are a swathe of pathologies associated with the sheath (e.g. neural sheath tumours, paragangliomas, metastatic lymph nodes, phlebitis, thromboembolism etc.), and understanding fascial anatomy of the sheath is crucial when planning surgical approaches and performing procedures such as carotid endarterectomy, internal jugular central venous line insertion and penetrating neck trauma reparation. To the best of our knowledge, there has been no comprehensive review of the carotid sheath anatomy, pathology, embryology and clinical significance. This review will therefore fill an important gap in literature by tying together and organising many complex anatomical descriptions and pathological and clinical accounts into one place, and will therefore be a very useful reference to both anatomists and clinicians.
2. Research methods in the literature
Methods used by investigators to explore the CS include dissection,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 fluid injection,1,6 radiographic imaging,18, 19, 20, 21, 22, 23, 24 clinical vignettes,7,12,25,26 serial sheet plastination,27,28 histology2,4,5,15,17,29, 30, 31, 32, 33 and literature reviews34, 35, 36, 37, 38 (Table 1).
Table 1.
Summary of literature on the carotid sheath (CS) selected for full-text analysis.
| Articles | Specimens | Age (Years old) | Sex | Sides | Study methods |
|---|---|---|---|---|---|
| Snosek et al (2020) | 12 cadavers | 78 ±; 55–98 | 6 M; 6 F | Both | Dissection; 0.9–1.2 cm thick sections |
| Feigl et al (2020) | 100 cadavers | 45–93 | 41 M; 59 F | N/A | Dissection; Thiel's embalmed |
| Hojaij et al (2019) | 50 cadavers | 68.51 ± 12.7 | 27 M; 23 F | Both | Surgical dissection with thyroidectomies |
| Komune et al (2019) | 2 whole and 19 sides of cadaveric heads | N/A | N/A | N/A | Microsurgical dissection |
| Bernard et al (2018) | 10 cadavers | N/A | N/A | N/A | Microsurgical dissection |
| Gavid et al (2018) | 10 cadavers | N/A | 7 M; 3 F | N/A | Microsurgical dissection; formalin and hydrogen peroxide fixed; Masson's Trichrome staining |
| Planitzer et al (2017) | 35 cadavers | 87.23 ± 6.72 | 14 M; 21 F | 34 left, 32 right | Dissection; ethanol-glycerin fixed |
| Katori et al (2013) | 15 fetuses | 28–37 weeks | N/A | N/A | Paraffin-embedded sections |
| Park et al (2013) | 18 fresh cadavers | 79.4 ± 6.9 | 10 M; 8 F | N/A | N/A |
| Lee et al (2012) | 2 cadavers | N/A | N/A | Both | Endoscopic dissection |
| Chaturvedi et al (2012) | 70 patients | 21–70 | 57 M; 13 F | Both | Intra-operative dissection |
| Miyake et al (2011) | 18 fetuses | 9–25 weeks | 6 M; 7 F | N/A | Horizontal (14) and sagittal (4) paraffin sections |
| Cavalcanti et al (2010) | 16 sides of cadaveric heads | N/A | 7 M; 9 F | Both | Dissection |
| Miyake et al (2010) | 15 fetuses | 9–25 weeks | 9 M; 6 F | Both | Horizontal paraffin sections |
| Froelich et al (2008) | 6 heads | N/A | N/A | Both | Dissection and histology examination |
| Hayashi (2007) | 5 cadavers | 76–9 | 2 M; 3 F | Both | Transverse and horizontal paraffin sections |
| Khafif-Hefetz et al (2004) | 34 patients | N/A | N/A | N/A | Dissection and histology |
| Zhang & Lee. (2002) | 6 cadavers | 54 to 86 | 2 M; 4 F | N/A | Plastinated slices |
| Piffer (1980) | 20 cadavers | N/A | N/A | Both | Microdissection |
| Gordinsky & Holyoke. (1938) | 75 cadavers; serial sections of 1adult cadaver and 5 fetuses | N/A | N/A | Both | ink injection; dissection |
| Barlow. (1936) | foetuses and adult specimen | N/A | N/A | N/A | Dissections, large serial sections and ink injection |
| Parson(s). 1910 | 4 cadavers | N/A | N/A | Right | Microscope sections |
2.1. Systematic review
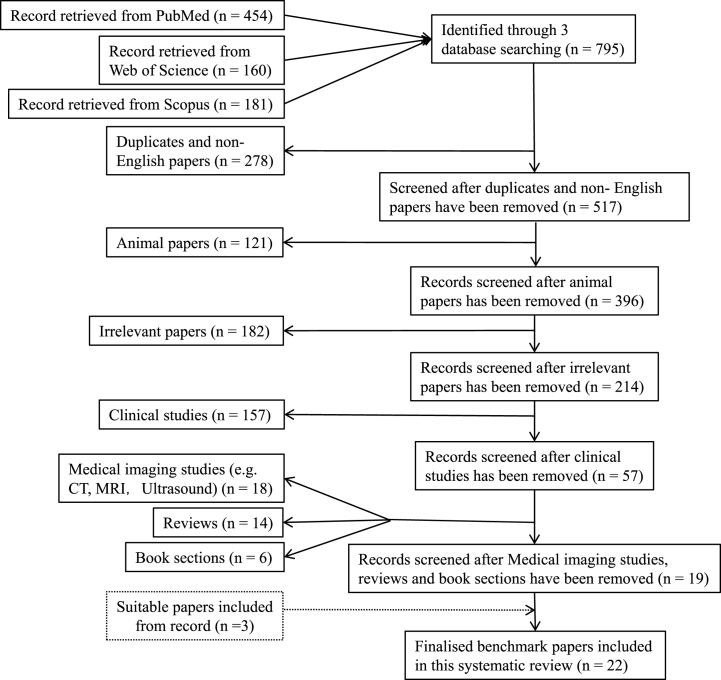
This part related to the fascial architecture of the CS to support the perspective of this review. Three databases (PubMed, Web of Science Core Collection) were searched on the 30th of August, 2021. Keywords in search of the title and abstract were “Anatomy AND Carotid Sheath”. The search results were limited to the English language. A total of 795 references including 454 from PubMed, 160 from Web of science and 181 from Scopus were retrieved and exported to EndNote reference management software (X9.3.3, Clarivate Analytics, London, UK). After removing duplicates (n = 226) and non-English articles (n = 52), 517 were left. To focus on direct evidence related to the anatomy of the human CS, further removals include animal studies (n = 121), irrelevant articles (e.g. studies in the lower limb, spine, internal organs, orbit) (n = 182), clinical studies (e.g. case reports, tumours, aneurysms, stroke, surgical methods) (n = 157), medical imaging studies (CT, MRI,Ultrasound) (n = 18), reviews (n = 14) and book sections (n = 6), 19 were retained and 3 additional articles which were not covered in the three databases were added. Thus, a total of 22 articles were for full-text reading and analysis (Table 1). Fig. 1 outlines the identification, screening, eligibility and included articles.
Fig. 1.
PRISMA flow chart displaying the steps in removing the search results. The horizontal arrow represents the number of papers removed upon each filtering. The vertical arrow represents number of papers that were kept from the previous removal.
3. Narrative review
3.1. Carotid sheath anatomy
3.1.1. Overview
Conventional descriptions organise the planes of the deep cervical fascia into three major layers. The superficial layer is the investing fascia which is located deep to the cervical subcutaneous tissue (or superficial fascia) and this layer is purported to envelop the neck as a whole. Embedded in this layer is the sternocleidomastoid muscle as it runs anteroinferiorly along the neck. The middle layer is the pretracheal fascia which surrounds the trachea, oesophagus, thyroid gland and suprahyoid and infrahyoid muscles. Lastly, the deep layer is the prevertebral fascia which covers the cervical spine and deep cervical muscles. Interposed between and abutting these three fundamental fascial layers is the carotid sheath (CS).36 The CS is a tubular column of deep cervical fascia originating from the skull base which courses along the prevertebral fascia behind the sternocleidomastoid muscle and then into the mediastinum behind the clavicle.2,11,12,14,26,32,34,38 Anterolateral to the CS is the investing fascia and the sternocleidomastoid muscle, the pretracheal fascia is anteromedial and the prevertebral fascia is posterior to the sheath. Regarding the anterolateral boundary of the deep cervical fascia, several authors define it by the paired carotid sheaths running through both sides of the neck.20,22,34 Similar to other deep fasciae, the fibrous tunnel that forms the CS is comprised of robust connective tissue and has no dedicated blood supply other than innominate branches from neighbouring vessels.2,3,12,34,38 Other names for this structure encountered in the literature include the great cervical sheath,23 vagina carotica,3 vascular sheath,5 carotid space (or pre-styloid space)18, 19, 20, 21, 22, 23 and post-styloid space23,36; the latter two are more prevalent in radiological studies and are not fasciae per se, rather they describe two sub-compartments of the parapharyngeal space.
3.1.2. Contents
Enclosed within the CS are the cervical great vessels, namely the common and internal carotid artery (ICA), internal jugular vein (IJV), and the vagus nerve3,9,12,14,29,32,33,38, 39, 40 (Table 2). The top of the CS also briefly transmits the lower cranial nerves (CNs) IX, XI and XII before they exit the sheath by piercing it anteriorly above the level of the hyoid.19, 20, 21, 22, 23 Kitamura (2018) states that instead of being contained inside the CS, CN IX (the glossopharyngeal nerve) in tandem with the ascending pharyngeal vessels, is embedded within the posterior wall of the CS.36 In addition to the major neurovascular triad, the CS contains fibres of a sympathetic plexus intermingled within the carotid artery adventitia, pericarotid venous plexuses and a chain of deep cervical lymph nodes. The ansa cervicalis (formed by C1-3 cervical spinal nerves) also runs across the anterior face of the CS to innervate the infrahyoid muscles.4,18, 19, 20, 21, 22, 23,34,38 The lymphatics associated with the CS are divided into two groups; the internal deep cervical nodes that run within the sheath, often adherent to the ICA intima,6,30,33 and the more numerous external deep lymph nodes, which include the jugulodigastric nodes; these nodes travel along the outer face of the CS.29,31 The facial, lingual, superior and middle thyroid veins all anastomose with the IJV as it passes down the CS and the pharyngeal and superior laryngeal nerves ramify off from the vagus nerve and exit the sheath.1,34
Table 2.
Summary of literature descriptions regarding the fasciae contributing to the carotid sheath (CS).
| Carotid sheath components | Author(s) | Description |
|---|---|---|
| Investing (superficial), pretracheal (middle) and prevertebral (deep) layers of the deep cervical fascia (DCF) |
Levitt (1970) | Carotid sheath has contributions from all the layers of the DCF |
| Paonessa and Goldstein (1976) | Superficial, middle and deep layers of the DCF contribute to the CS with involvement of the alar fascia | |
| Harnsberger and Osborn (1991) | All three layers of the DCF contribute to and circumscribe the CS | |
| Bielamowicz et al (1994) | All three layers of the DCF form the CS | |
| Kuwada et al (2012) | The CS is encircled by the 3 layers of the DCF | |
| Guidera et al (2014) | The CS is composed of the 3 layers of the DCF | |
| Garner and Baker (2018) | The CS is composed of the 3 layers of the DCF | |
| Sutcliffe and Lasrado (2020) | Fibres from all three layers of the DCF blend with the carotid sheath | |
| Chengazi & Bhatt (2019) |
Anterolateral CS wall is made by the superficial fascia, the anteromedial wall by the middle fascia and the posterior wall by the DCF |
|
| Superficial and middle cervical fasciae only |
Hollinshead (1968) | The superficial and pretracheal fascia form the anterolateral CS wall while a medially reflection of the superficial fascia forms the posterior and medial wall |
| Anderson and Homan (2008) | The CS has components of the investing and pretracheal fascia | |
| Miyake et al (2010) |
Parts of the CS are composed of the superficial and pretracheal cervical fasciae |
|
| Middle cervical fascia only |
Natale et al (2015) |
The visceral fascia in particular forms the CS |
| Deep cervical fascia only |
Gray and Carter (1858) | The deep layer of the DCF assists in forming the CS |
| Mosher (1920) |
The CS is made from the deep (prevertebral) cervical fascia |
|
| Deep cervical + other fascia combination |
Gray (1918) | The strong CS is derived from the DCF and the fascia lining the deep surface of the sternocleidomastoid muscle |
| Barlow (1936) |
The deep fascia completes the CS posteriorly while the stylopharyngeal aponeurotic fascia seals off the sheath anteriorly |
|
| None of the three cervical fasciae |
Grodinsky & Holyoke (1938) | CS is comprised of alar fascia with layers of reinforcement from the sternothyroid and sternocleidomastoid muscle sheathes |
| Komune et al (2019) |
CS is a fibrous network created by fascia from the longus capitis and stylopharyngeal muscles, plus the buccopharyngeal and tensor-vascular styloid fasciae |
|
| No fascial contribution | Parsons (1910) | No discrete fascial tube surrounding the ICA, IJV or vagus nerve |
| Katori et al (2013) | No continuous sheath encircling the CS neurovasculature |
The CS serves as a conduit that protects its traversing neurovascular contents and sequesters them into a single distinct cervical compartment. Furthermore, it aids the passage of these structures through the neck and forms a barrier against spread of infection from the neck into the sheath or vice versa.33,34
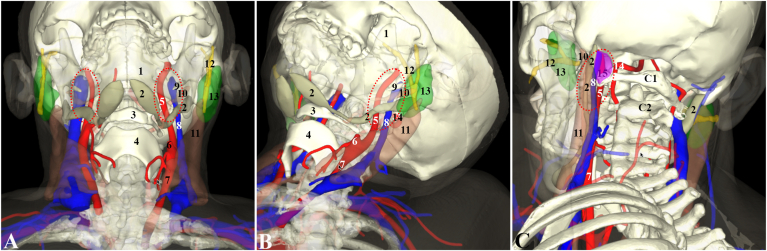
Sparse areolar connective tissue fills the space between the ICA, IJV and vagus nerve and supports the nervous plexuses and also a small venous pericarotid plexus.4,11 Bond et al (2020) describe a pocket of adipovenous tissue found at the external opening of the jugular foramen which was identified as an extracranial prolongation of the extradural neural axis compartment (EDNAC) into the superior CS. It was interposed between the anteromedial ICA and posterolateral IJV and contained the lower cranial nerves (CNs IX – XII) although how far down this fatty tissue may extend within the sheath was not explored.27 On medical images, however, the neighbouring vascular and bony structures could be used to localize it. Fig. 2 is the 3D reconstruction image from CT scans of a 59 years old male with chronic tonsillitis, showing the main vascular contents and the neighbouring main landmarks of the left upper CS.
Fig. 2.
Three dimensional reconstruction of the left upper carotid sheath and its surrounding main landmarks from CT scans of a 59 years old male with chronic tonsillitis. A and B are the anterior view and anterolateral view with about 15° neck extension, whereas C is a posterolateral view. The mandible (1) is presented with 30% opacity. 1: mandible, 2:digastric muscle, 3: hyoid, 4: thyroid cartilage, 5: internal carotid artery, 6: external carotid artery, 7: common carotid artery, 8: internal jugular vein, 9: styloid process, 10: stylohyoid muscle, 11: sternocleidomastoid muscle, 12: facial nerve (in and distal to parotid gland), 13: parotid gland, 14: vertebral artery, 15: indicating the location of the rectus capitis lateralis, C1: 1st cervical vertebra; C2: 2nd cervical vertebra.
3.1.3. Fascial tubes
A number of studies have noted the existence of neurovascular subcompartmentation within the CS. Distinct fibrous tunnels ensheath the ICA, IJV and vagus nerve into their own compartments. However, Feigl et al (2019) express uncertainty regarding their extent.1,3,6,9,11,12,15 The wrapping of the CS around the IJV was noted by Barlow (1936) to be quite loosely adhered to the venous adventitia.1 Gray's Anatomy (1858) described fibrous septa partitioning the CS contents whereas Gray's (1918) opt to define the vessels and nerve as invested in their own sub-sheaths.39,40 A brief review of the French, Spanish and Italian literature by Piffer (1980) supports this sub-sheath anatomical arrangement.15 The same author reports an additional small sheath for the glossopharyngeal nerve (CN IX) in the superior CS.15 Loose connective tissue tethers the internal wall of the CS to both the ICA and IJV and the vagus nerve, which possess a notably slight and delicate sheath, is fused posteriorly to the sheathes of the artery and vein.2,4,6,15 A pleated fold in the posterior CS surrounds the intra-carotid cervical sympathetic plexus.6,12,22 Hollinshead (1968), Hayashi (2007) and Chaturvedi et al (2012) contradict this however, and state that the sympathetic plexus and ganglion reside in the prevertebral fascia running posterior to the CS.2,9,29
3.1.4. Spatial arrangement of CS contents
The consensus is that the ICA courses medially within the CS with the IJV lying lateral to the artery. Chengazi & Bhatt (2019) conversely describe the ICA as being centrally positioned in the CS with the IJV residing posterolaterally.20 The adjoining vessels create a groove posteriorly which is generally occupied by the vagus nerve, although many variations in its position have been observed.2,20,22,34,40 A dissection study by Hojaij et al (2019) found that 64% of right-sided vagus nerves were positioned behind the vessels, however 68% of left-sided vagus nerves passed anterior to the vessels. Very few cases displayed symmetry of vagus nerve position between left and right sheaths.8 Cunningham and Martínez (2021) in their review of the literature present ten different spatial arrangements of the vagus nerve within the CS. In eight of the variations, the vagus nerve is situated at some point around the ICA, while for the remaining two variations, the nerve is lateral or posterolateral to the IJV.41 Additionally, a rare case where the right IJV was positioned medial to the ICA was reported by Shoja et al (2007).24
3.1.5. Attachments of the CS
There is disagreement on the precise superior and inferior attachments of the CS.3 Many authors state that the upper CS simply attaches to the skull base7,18,21,22,34 while some pinpoint its insertion around the mouth of the external jugular foramen (JF), specifically the posterior rim.2,10,15,20,23 Anteriorly, the CS adheres to the external carotid canal.15 Lateral to the JF and interposed between the temporomandibular fossa and styloid process protrudes the vaginal process (VP), an important but controversial landmark for the CS. Froelich et al (2008) report the CS attaching laterally to the VP, Piffer (1980) describes a medial attachment and Komune et al (2019) conversely state that the CS attaches to the anterior face of the VP.4,10,15 The CS sends out fibres to intermingle with thick fibrocartilage that bridges and encircles the extracranial openings of both the jugular foramen and carotid canal.2,4,10 The CS is attested to be a continuation of extracranial periosteum by Froelich et al (2008) and Piffer (1980) but according to Sutcliffe & Lasrado (2020) it is continuous with the intracranial dura mater.4,15,38
As the CS travels craniocaudally down the neck it is held in place by multiple fascial attachments, medially to the retropharyngeal fascia (e.g. buccopharyngeal and alar fascia) and posteriorly to the prevertebral fascia. All of the three deep cervical fascial layers blend with the CS in the root of the neck.9,12 Guidera et al (2013) imply that the CS is attached to the omohyoid fascia in the mid-neck region.35 The anterior pretracheal fascia does not associate with the CS, and there exists a small gap of 0.5–2 mm between the structures.29 Grodinsky and Holyoke (1938) report that in the upper neck, the CS runs deep to and is fused with the anteroinferior sheaths of the stylohyoid and posterior belly of the digastric muscle.6 Thus, the upper CS may contain those musculotendinous components.
At the thoracic inlet and within the upper mediastinum, the CS is said to insert onto the aortic arch.7,18,20, 21, 22, 23 It has also been observed that the inferior CS is linked to the fibrous pericardium via the visceral fascia which bridges these two structures.6 Other studies assert that the CS becomes apparent at the level superior to the first rib, sternum and clavicle with the anterior sheath adhering to the latter two bones.6,11,12,34 Grodinsky and Holyoke (1938) note that the CS also fuses with the suprapleural membrane (Sibson's fascia) immediately superficial to the cupula of the lung.6 The CS then trifurcates in the thorax into three separate sleeves containing the IJV, ICA and vagus nerve as they travel along their respective routes.6,11
3.1.6. Zoning of the CS
The hyoid bone is used to subdivide the CS into two parts along its length. The suprahyoid CS is also known as the poststyloid compartment of the parapharyngeal space.18, 19, 20, 21, 22 The contents of these two parts are essentially the same except that the lower cranial nerves (CNs IX – XII) pass through the suprahyoid CS and the common carotid artery resides in the infrahyoid CS.20,21 Chaturvedi et al (2012) describe additional subdivisions in the upper suprahyoid CS that are associated with CNs IX, XI and XII.2 The CS runs through the carotid triangle in the superficial neck which overlaps with both the suprahyoid and infrahyoid compartments.19 Digressing from this two-part arrangement, Hayashi (2007) organises the CS into three segments; an upper (from the hyoid bone up), a middle (from the larynx/thyroid gland to the hyoid) and a lower (from the subclavian artery to the larynx/thyroid gland).29 Fluid injected into the infrahyoid CS tends to remain localised below the hyoid bone and above the root of the neck, suggesting that there is limited communication between the upper and lower CS levels. Its structural basis remains unclear and may be influenced by the fibro-adipose areolar tissue filling the spaces between the neurovascular bundle,6,9 and/or its fusion with the musculotendinous sheaths of the stylohyoid and digastric muscles.6
3.1.7. Fascial components of the CS
One of the most controversial aspects of the CS relates to the fascia that constitutes this structure. Similar to descriptions of other fasciae in the body, there are multiple different standpoints and claims regarding the numerous fasciae contributing to the CS. These can be organised into three main categories, from which a consensus can be drawn that the CS has multiple origins.
The majority of accounts report that the CS is formed from all three layers of the deep cervical fascia i. e, the investing (superficial), pretracheal (middle) and prevertebral (deep) fasciae.11,13,19,21,22,26,34,35,38,40 Chengazi & Bhatt (2019) specify that the anterolateral CS wall is made by the investing fascia, the anteromedial wall by the pretracheal fascia and the posterior wall by the prevertebral fascia.20 Both the investing and pretracheal fasciae are said by Hollinshead (1968) to form the anterolateral CS wall. The same author claims that the posterior and medial CS wall is formed by a medially reflected lamina from the investing fascia.9
The second category involves studies who mention only one or two of the deep cervical fasciae forming the CS i.e. the investing (superficial) and pretracheal (middle),18,32 the pretracheal (middle)18,37 or the prevertebral (deep) fascia.1,39
The third category describes authors who assert an origin of the CS that does not involve any of the three cervical fasciae. According to Grodinsky & Holyoke (1938), the CS is comprised of alar fascia with layers of reinforcement from the sternothyroid and sternocleidomastoid muscle sheathes.6 Another idea is that the CS is created by strong fascial aponeurosis from muscle sheathes. Gray's Anatomy 20th edition (1918) states the sheath derives from the sternocleidomastoid whereas Barlow (1936) hold the view that it originates from the exceedingly strong stylopharyngeal aponeurosis.1,40 Lastly, Komune et al (2019) uniquely report that multiple thin fasciae from the longus capitis and stylopharyngeal muscles, plus the buccopharyngeal and tensor-vascular styloid fasciae all coalesce to form a fibrous network that enveloped the neurovascular bundle of the neck.10
Regarding the alar fascia (AF), a number of authors credit this fascia as an important contributor to the CS. The composition of the AF (or fascia alaris) is debated; it is described as thick,3 very thin,5 delicate17 and multilaminar in nature.29 The fibroareolar AF resides between the prevertebral fascia and the anterior deep cervical muscles (the longus capitis and longus cervicis muscles), and extends laterally in a coronal plane to fuse with the CS, thereby linking both CSs bilaterally.5,10,17,29 At the midline, the AF is associated with the prevertebral and visceral fasciae and is also attached to the bony transverse processes of the cervical vertebrae.3,5, 6, 7,10,13,29 Laterally, the AF primarily acts to form the medial wall of the CS and sends fibres to reinforce the anteromedial wall, and, to a lesser extent, the posteromedial wall of the sheath.3,5,7,10,13,17 Feigl et al (2019) refer to the AF as the ‘intercarotid fascia’ (or fascia intercarotica) and report it as partially thick, dense regular connective tissue which is fused anteriorly to the buccopharyngeal fascia in the midline in around 50% of cases.3
3.1.8. Models of the CS
Hayashi (2007) reviewed the literature on anatomical models of CS fascia arrangement in the neck.29 The following six descriptions refer to the sectional CS shape in the neck when viewed in a transverse plane. The first model by Hollinshead (1982) presents a very large, triangular CS, and is the only model to depict subcompartmentation of the neurovascular structures with abundant surrounding space.9 The anterior and lateral points of the triangle extend deep to the sternocleidomastoid and omohyoid muscles respectively. Spitzka (1913) shows another triangular CS, of a smaller area than Hollinshead (1982), with extension of just the posterolateral edge back along the omohyoid muscle. The CS is similarly sized and teardrop-shaped in the third model by Clemente (1985) with the tail of the teardrop extending anteriorly underneath the sternocleidomastoid muscle. Ouchi (1982) shows a small CS that is fused along part of the sternocleidomastoid and omohyoid muscles laterally, and is rounded on the medial aspect, around the ICA. The fifth and sixth models by Grodinsky and Holyoke (1938) and Hayashi (2007) have the smallest area, with the CS closely circumscribing the vessels and nerve, and are roughly circular in shape.9
3.1.9. Double sheath
The CS was subdivided by Hayashi (2007) into two sub-sheathes (or laminae) entailing a thick inner ring comprising multi-layered, adventitial tissue encasing the ICA and IJV and a thin, outer ‘common sheath’ of tough, fibrous connective tissue.29,32 The vagus nerve was interposed between these two laminae.29 In a similar vein, Grodinsky & Holyoke (1938) propose that there are interrupted double layers in the anteromedial and anterolateral sheath where the CS fuses with fascia coming off the sternothyroid and sternocleidomastoid muscles.6
3.1.10. Histology and embryology of the CS
Histological investigations have shown that the CS is made up of fibrous, elastic, varying amounts of adipose tissue with small nerves and microvessels contained within the sheath wall.2,33 The CS contains circular and oblique collagen fibres and there is a clearly demarcated plane of division between the CS outer wall and the surrounding cervical tissue.2,15
During development, the CS forms from mesenchymal tissue derived from the embryonic mesoderm.16,34 The distinct adventitia of the ICA and IJV is the first to develop, at 15 weeks, and the CS becomes apparent later on, condensing around the vessels at 20 weeks.32,34 In the early stages of its formation, the CS is fused to the pretracheal fascia, but separated from the prevertebral fascia and there is a neighbouring lamina of fatty-lymphoid tissue.32,34,35 From weeks 9–20, the vagus nerve also migrates from a ventral position in the sheath, across to the lateral CS wall.32
3.1.11. Spatial anatomy around the CS
The suprahyoid CS is intimately related to a number of structures in the neck, particularly the cervical spaces. Anteriorly, the CS is bordered by the prestyloid parapharyngeal space, styloid process and masticator muscle.19, 20, 21,23,36 Several authors classify the poststyloid parapharyngeal compartment as the carotid space, occupied by the CS and its contents.7,18,22 The sternocleidomastoid muscle and stylopharyngeal and interpterygoid fasciae pass anterolaterally to the CS, and the parotid space and digastric muscle (anterior belly) reside laterally.10,19,20,22,23 On the medial side of the sheath are the retropharyngeal, danger and visceral spaces and the anteromedial wall of the pharynx.18, 19, 20,22,23 Posterior to the CS are perivertebral, prevertebral, pretracheal and posterior cervical spaces as well as the prevertebral fascia, longus capitis, rectus capitis lateralis and lateral atlanto-occipital ligament.10,19,20,22,36
Below the submandibular space and styloglossus and stylopharyngeus muscles, the CS is traditionally understood to be completely sandwiched between the investing, pretracheal and prevertebral layers of the deep cervical fascia, however, Zhang & Lee (2002) made a fascinating discovery regarding the investing fascia at the site of the trapezius and sternocleidomastoid muscles.28,36 Their investigation revealed an absence of a fibrous lamina covering the gap between these two muscles, and that the CS is actually in direct communication with subcutaneous tissue in the neck via a connecting corridor of fatty, areolar tissue extending from the lateral CS wall and out between the two muscle bellies.28,32
At the skull base is the sizable pharyngomaxillary fossa (or space), which is positioned like an inverted cone; an early study by Mosher (1920) argues that this fossa receives the ICA, IJV and vagus nerve, and that the CS is essentially a cervical continuation of this space.26 Building on this idea is the trifolium theory proposed by Beck (1933) which involves the parotid, submaxillary and pharyngomaxillary spaces.25 These three spaces are said to directly communicate with the CS inferiorly, thus forming a trifolium; a ‘three-leafed’ arrangement with the superior three spaces forming the ‘leaves’ and the CS the ‘stalk’ similar to the clover (or trefoil) plant.25 The existence of this anatomical arrangement was denied by Barlow (1936) who observed that any communication between the CS and pharyngomaxillary space was obstructed by the extremely robust stylopharyngeal aponeurosis.1
3.1.12. Anatomical variations of the CS
As is the case with many anatomical structures, minor variations in the CS anatomy have been observed. The CS varies in thickness along its length, being thinner in the upper and lowermost regions, and also between individuals.2,15,33,35 Hayashi (2007) mention that the CS may be incomplete in some regions, and Garner et al (2020) describe how the abnormal common carotid arteries can distort the spatial position of the CS.29,34
Most authors consider the CS to be present as a definite fascial condensation wrapping around the neurovascular bundle and this has been observed both in dissections and histologically.1,6,11,15,16,29,32,35 The denial of a CS, especially in papers from the 19th century was retold in Grodinsky & Holyoke (1938) and in an early dissection study by Parsons (1910), the author could identify no discrete fascial tube surrounding the ICA, IJV or vagus nerve, but rather observed these structures embedded in a padding of loose, areolar tissue.6,14
4. Pathologies of the CS
4.1. Infectious spread
The carotid sheath is subject to numerous pathologies, reflecting the variety of structures contained within it. It is rare to observe primary pathologies arising from the CS itself and most problems are related to disorders of the sheath's neural, vascular and lymphatic contents.23,33,34 Of clinical importance is the visceral-vascular space9,11,13 which is the small potential space within the CS, analogous to the retrovisceral space that can act as a conduit to facilitate infection spread from the neck to the superior mediastinum.9,25,36 Levitt (1970) contends that infections within the sheath are unlikely to spread, but rather remain localised.11 Such infections usually originate from dental abscesses or the submaxillary or parotid compartments which then spread into the pharyngomaxillary space and prestyloid and poststyloid spaces. The infection can then spread into tympanic membrane and mastoid process, and the parapharyngeal space which consists of the prestyloid and poststyloid (or the CS).13,22 Via the parapharyngeal space, all of the deep neck spaces can become involved and contribute to infection spread along the CS and into the mediastinum.22 Such odontogenic or deep cervical infection can induce suppurative jugular thrombophlebitis (Lemierre Syndrome), a serious condition where pus containing typically Fusobacterium necrophorum bacteria seeps into the IJV, leading to septicaemia and septic embolism formation.20,34
4.2. Nervous pathologies
Tumours associated with the CS are organised into metastatic and primary. The majority of cervical metastases are from squamous cell carcinomas and these can seed neoplasms into the deep internal and external parajugular lymph nodes which can lead to enlarged lymph nodes, the most common CS pathology.19,20,22,23,30 Primary lesions of the CS are rare but numerous in their origins and can be identified by their anterior displacement of the parapharyngeal space.21 Paragangliomas of the carotid space and can be subdivided into i) carotid body tumours, ii) glomus jugulare tumours along Jacobson's nerve (CN IX) or Arnold's nerve (auricular branch of CN X) and iii) glomus vagale tumours along CN X which can invade the jugular foramen superiorly and displace the ICA anteriorly.20,22 Other tumour types include nerve sheath tumours such as schwannomas, neurofibromas and neuroblastomas (the commonest CS malignancy in children).19,20,23 Intracranial meningiomas can also extend into the carotid space from the jugular foramen, utilising the EDNAC adipose tunnel, and there are very rare instances of primary extradural meningiomas within the CS.20,27
4.3. Vascular pathologies
The ICA and IJV within the CS are prone to several disorders such as pseudotumours (caused by a tortuous ICA), and a number of vascular lesions.22 Arterial lesions include dissection of the ICA causing lumen stenosis, carotid pseudoaneurysm which forms a heamatoma in the vascular wall, atherosclerotic plaque formation of the ICA intima (especially at the carotid bifurcation), thrombus formation within the ICA, carotidynia (Fay syndrome) which exhibits as localised pain at the carotid bifurcation, ICA erosion due to infection and fibromuscular dysplasia caused by a hyperplastic tunica media.20,34,42 Additionally, the IJV can become inflamed (phlebitis) during septicaemia or eroded following localised infections and can also be the site for thrombosis development following a number of precipitants.13,20,42
4.4. Surgical implications
As noted by Levitt (1970), the carotid sheath is particularly clinically significant due to its relationships and connections with virtually all of the cervical fascia.11 Due to its robust fascial composition, the CS can form a barrier against metastatic spread within the neck.35 Due to its thickness, exposing the CS contents (e.g. ICA) is challenging when accessing the area laterally using the Fisch, preauricular, subtemporal or infratemporal approaches.4 As discussed previously, the significant variation in vagus nerve position in relation to the IVJ and ICA is important to consider during operations and is relevant for neural monitoring during a thyroidectomy for example.8 The direct communication of the CS with the cervical subcutaneous tissue as described by Zhang and Lee (2002) serves as a useful anatomic corridor for anterolateral surgical access of the sheath itself and its contents, as well as the deep cervical lymph nodes and retropharyngeal or parapharyngeal spaces.28 Two routine clinical procedures associated with the CS are carotid endarterectomies for atherosclerotic plaques and central venous line access into the IJV for the administration of IV medication or fluids.34 Clinical manifestations of the aforementioned phlebitis or inflammation of the carotid vascular space include septicaemia and spiking fevers, persistent tenderness on palpation, contralateral torticollis and localised swelling (although the latter may also be caused by inflamed lymph nodes).11,25
Because the CS contributes significantly to the fascial component of the neck and extracranial skull base, it is very important to consider when operating in those regions. As an integral part of the deep cervical fasciae, the CS is intimately associated with the visceral compartment anteriorly and vertebral compartment posteriorly, and importantly, services as a communicating pathway between the intracranial (i.e. the EDNAC) and extracranial spaces (e.g. the parapharyngeal space and retropharyngeal space) along its upper and lower levels. As described previously, the carotid space can hence be utilised as a route for infectious spread or tumour metastasis and encroachment. Various surgical approaches are closely associated with the carotid sheath, particularly endoscopic approaches (Table 3).43,44 Fig. 2A, B and 2C represent the visualization of the upper CS in the anterior, anterolateral and posterolateral surgical approaches. From a posterior or lateral approach perspective, the rectus capitus lateralis (RCL) is an important anatomical landmark (Fig. 2C) for identifying nerves and vessels emerging from the jugular foramen within the upper CS.45 In terms of the intricate parapharyngeal space (PPS) which abuts with the CS, masses can develop in this area (around 0.5% of all head and neck tumours) and negotiating around the CS with its crucial neurovascular contents needs to be performed carefully.44,46
Table 3.
Surgical approaches and landmarks for the upper carotid sheath (CS).
| Approaches | Main Landmarks | Other landmarks | |
|---|---|---|---|
| Oral approach |
Open | Styloid process and associated structures. |
Superior pharyngeal constrictor |
| Endoscopic or Robotic |
Medial pterygoid; superior pharyngeal constrictor |
||
| Cervical approach |
Prevertebral lateral cervical | Posterior belly of digastric muscle |
Hyoid greater horn, hyoglossus, longus capitis, longus colli, superior pharyngeal constrictor, anterior tubercle of atlas |
| Cervical - parotid | Facial nerve | ||
| Cervical - mandible |
Styloglossus, stylopharyngeus |
||
| Cervico-cranial approach | Postero-lateral | Lateral rectus capitis | Digastric crest, posterior rectus major, superior oblique, inferior oblique, occipital condyle, jugular process |
| Sub-temporal | Transverse process of atlas | Longissimus capitis, posterior abdomen of digastric muscle, root of styloid process and jugular process | |
| Retro-auricular | Transverse process of atlas | Digastric crest, facial canal, occipital condyle, jugular nodule, rectus capitis, jugular vein process | |
5. Conclusion
The CS has been shown to be an integral yet complicated component of the deep cervical fascia. Aspects of its anatomy are still controversial and it is subject to a plethora of albeit rare pathologies which, from a clinical relevance standpoint, require a sound understanding of the CS structure, extent and arrangement of its neurovascular contents to ensure adequate outcomes following surgery. This review has provided an overview of the key anatomical, pathological and clinical aspects pertaining to the CS and highlighted the issues that can be elucidated by future surgical anatomy studies to clarify the discrepancies herein described regarding the intricate anatomy of the CS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present study was funded by a University of Otago research grant (2018-2020), the National Natural Science Foundation of China (grant 81671368) and the Project of Hefei City, China (Project No. J2020Y05)
Abbreviations and acronyms
- CS
carotid sheath
- ICA
internal carotid artery
- IJV
internal jugular vein
- CN
cranial nerve
- EDNAC
extradural neural axis compartment
- JF
jugular foramen
- VP
vaginal process
- AF
alar fascia
- RCL:
rectus capitis lateralis
- PPS
parapharyngeal space
References
- 1.Barlow D. The surgical anatomy of the neck in relation to septic lesions: an investigation of the cervical connective tissue. J Anat. 1936;70(Pt 4):548. [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi P., Vaishampayan S.S., Nair S., Nair D., Pawar P., Kane S. Routine removal of the carotid sheath as part of neck dissection is unnecessary if grossly uninvolved as seen intra-operatively. Int J Oral Maxillofac Surg. May 2012;41(5):576–580. doi: 10.1016/j.ijom.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Feigl G., Hammer G.P., Litz R., Kachlik D. The intercarotid or alar fascia, other cervical fascias, and their adjacent spaces - a plea for clarification of cervical fascia and spaces terminology. J Anat. Jul 2020;237(1):197–207. doi: 10.1111/joa.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froelich S.C., Aziz K.M.A., Levine N.B., Pensak M.L., Theodosopoulos P.V., Keller J.T. Exposure of the distal cervical segment of the internal carotid artery using the trans-spinosum corridor: cadaveric study of surgical anatomy. Neurosurgery. May 2008;62(5):354–361. doi: 10.1227/01.Neu.0000310761.83992.24. [DOI] [PubMed] [Google Scholar]
- 5.Gavid M., Dumollard J.M., Habougit C., et al. Anatomical and histological study of the deep neck fasciae: does the alar fascia exist? Surg Radiol Anat. Aug 2018;40(8):917–922. doi: 10.1007/s00276-018-1977-5. [DOI] [PubMed] [Google Scholar]
- 6.Grodinsky M., Holyoke E.A. The fasciae and fascial spaces of the head, neck and adjacent regions. Am J Anat. 1938;63(3):367–408. [Google Scholar]
- 7.Hall C. LXIV. The parapharyngeal space: an anatomical and clinical study. Ann Otol Rhinol Laryngol. 1934;3:793–812. [Google Scholar]
- 8.Hojaij F., Rebelo G., Akamatsu F., et al. Syntopy of vagus nerve in the carotid sheath: a dissectional study of 50 cadavers. Laryngoscope Investigative Otolaryngology. Jun 2019;4(3):319–322. doi: 10.1002/lio2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollinshead W.H. LWW; 1968. Anatomy for Surgeons: Volume 1—the Head and Neck. [Google Scholar]
- 10.Komune N., Matsuo S., Nakagawa T. The fascial layers attached to the skull base: a cadaveric study. World Neurosurgery. Jun 2019;126:E500–E509. doi: 10.1016/j.wneu.2019.02.078. [DOI] [PubMed] [Google Scholar]
- 11.Levitt G.W. Cervical fascia and deep neck infections. Laryngoscope. 1970;80(3):409–435. doi: 10.1288/00005537-197003000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lindner H.H. The anatomy of the fasciae of the face and neck with particular reference to the spread and treatment of intraoral infections (Ludwig's) that have progressed into adjacent fascial spaces. Ann Surg. 1986;204(6):705. doi: 10.1097/00000658-198612000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paonessa D.F., Goldstein J.C. Anatomy and physiology of head and neck infections (with emphasis on the fascia of the face and neck) Infectious diseases of the head and neck. Otolaryngol Clin. 1976;9(3):561–580. [PubMed] [Google Scholar]
- 14.Parsons F. On the carotid sheath and other fascial planes. J Anat Physiol. 1910;44(Pt 2):153. [PMC free article] [PubMed] [Google Scholar]
- 15.Piffer C.R. Mesoscopic and microscopic study of the carotid sheath. Acta Anat. 1980;106(4):393–399. doi: 10.1159/000145207. [DOI] [PubMed] [Google Scholar]
- 16.Snitman M.F., Soboroff B.J. Planes of surgical dissection of the neck. Ann Otol Rhinol Laryngol. 1956;65(1):49–56. doi: 10.1177/000348945606500105. [DOI] [PubMed] [Google Scholar]
- 17.Snosek M., Macchi V., Stecco C., Tubbs R.S., De Caro R., Loukas M. Anatomical and histological study of the alar fascia. Clin Anat. 2020;34(4):609–616. doi: 10.1002/ca.23644. May. [DOI] [PubMed] [Google Scholar]
- 18.Anderson J.C., Homan J.A. Radiographic correlation with neck anatomy. Oral Maxillofac Surg Clin North Am. 2008;20(3):311–319. doi: 10.1016/j.coms.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Bielamowicz S.A., Storper I.S., Jabour B.A., Lufkin R.B., Hanafee W.N. Spaces and triangles of the head and neck. Head Neck. 1994;16(4):383–388. doi: 10.1002/hed.2880160415. [DOI] [PubMed] [Google Scholar]
- 20.Chengazi H.U., Bhatt A.A. Pathology of the carotid space. Insights Imaging. 2019;10(1):21. doi: 10.1186/s13244-019-0704-z. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harnsberger H.R., Osborn A.G. Differential diagnosis of head and neck lesions based on their space of origin. 1. The suprahyoid part of the neck. AJR American J Roentgenol. 1991;157(1):147–154. doi: 10.2214/ajr.157.1.2048510. [DOI] [PubMed] [Google Scholar]
- 22.Kuwada C., Mannion K., Aulino J.M., Kanekar S.G. Imaging of the carotid space. Otolaryngol Clin North Am. 2012;45(6):1273–1292. doi: 10.1016/j.otc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Meesa I.R., Mukherji S.K. A simplified approach to the spaces of the suprahyoid neck. Appl Radiol. 2017;46(4):6–14. doi: 10.1016/s0033-8389(05)70063-4. [DOI] [PubMed] [Google Scholar]
- 24.Shoja M.M., Tubbs R.S., Ardalan M.R., Jalilvand R., Oakes W.J. Right medial internal jugular vein: a reversed carotid sheath. Italian J Anat Embryol= Archivio italiano di anatomia ed embriologia. 2007;112(4):277–280. [PubMed] [Google Scholar]
- 25.Beck A.L. A study of twenty-four cases of neck infection. Ann Otol Rhinol Laryngol. 1933;42:741. [Google Scholar]
- 26.Mosher H.P. Deep cervical abscess and thrombosis of the internal jugular vein. Laryngoscope. 1920;30(6):365–375. [Google Scholar]
- 27.Bond J.D., Xu Z., Zhang M. Fine configuration of the dural fibrous network and the extradural neural axis compartment in the jugular foramen: an epoxy sheet plastination and confocal microscopy study. J Neurosurg. 2020;1:1–11. doi: 10.3171/2020.4.JNS20811. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M., Lee A.S. The investing layer of the deep cervical fascia does not exist between the sternocleidomastoid and trapezius muscles. Otolaryngol Head Neck Surg. 2002;127(5):452–454. doi: 10.1067/mhn.2002.129823. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi S. Histology of the human carotid sheath revisited. Okajimas Folia Anat Jpn. 2007;84(2):49–60. doi: 10.2535/ofaj.84.49. [DOI] [PubMed] [Google Scholar]
- 30.Khafif-Hefetz A., Leider-Trejo L., Medina J.E., Gil Z., Fliss D.M. The carotid sheath: an anatomicophathologic study. Head Neck. Jul 2004;26(7):594–597. doi: 10.1002/hed.20021. [DOI] [PubMed] [Google Scholar]
- 31.Kolomvos N., Skouteris C.A., Papadogeorgakis N., Sklavounou A., Alexandridis C., Angelopoulos A.P. Histopathologic study of the carotid sheath in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. Oct 2010;68(10):2452–2458. doi: 10.1016/j.joms.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Miyake N., Hayashi S., Kawase T., et al. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Record-Adv Integ Anat Evol Biol. 2010;293(3):438–445. doi: 10.1002/ar.21089. [DOI] [PubMed] [Google Scholar]
- 33.Palliyalil M., Anehosur V., Joshi A., Acharya S. Histological assessment of the carotid sheath in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75(11):2465–2476. doi: 10.1016/j.joms.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Garner D.H., Kortz M.W., Baker S. 2018. Anatomy, Head and Neck, Carotid Sheath. [Internet; Bookshelf ID: NBK519577] [PubMed] [Google Scholar]
- 35.Guidera A.K., Dawes P.J., Fong A., Stringer M.D. Head and neck fascia and compartments: no space for spaces. Head Neck. 2014;36(7):1058–1068. doi: 10.1002/hed.23442. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura S. Anatomy of the fasciae and fascial spaces of the maxillofacial and the anterior neck regions. Anat Sci Int. 2018;93(1):1–13. doi: 10.1007/s12565-017-0394-x. [DOI] [PubMed] [Google Scholar]
- 37.Natale G., Condino S., Stecco A., Soldani P., Belmonte M.M., Gesi M. Is the cervical fascia an anatomical proteus? Surg Radiol Anat. 2015;37(9):1119–1127. doi: 10.1007/s00276-015-1480-1. [DOI] [PubMed] [Google Scholar]
- 38.Sutcliffe P., Lasrado S. Anatomy, head and neck, deep cervical neck fascia. StatPearls [Internet]; 2020. [PubMed] [Google Scholar]
- 39.Gray H., Carter H.V. Grange Books; 1858. Gray's Anatomy: A Facsimile. [Google Scholar]
- 40.Gray H. Lea and Febiger; Philadelphia: 1918. Henry Gray's Anatomy of the Human Body. [Google Scholar]
- 41.Cunningham C.J., Martínez J.L. The wandering nerve: positional variations of the cervical vagus nerve and neurosurgical implications. World Neurosurgery. 2021;156:105–110. doi: 10.1016/j.wneu.2021.09.090. [DOI] [PubMed] [Google Scholar]
- 42.Mosher H.P. The submaxillary fossa approach to deep pus in the neck. Trans Am Acad Ophthalmol Otolaryngol. 1929;34:19–26. [Google Scholar]
- 43.Anichini G., Evins A.I., Boeris D., Stieg P.E., Bernardo A. Three-dimensional endoscope-assisted surgical approach to the foramen magnum and craniovertebral junction: minimizing bone resection with the aid of the endoscope. World Neurosurg. 2014;82(6):e797–e805. doi: 10.1016/j.wneu.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Strohl M.P., El-Sayed I.H. Contemporary management of parapharyngeal tumors. Curr Oncol Rep. 2019;21(11):1–5. doi: 10.1007/s11912-019-0853-8. [DOI] [PubMed] [Google Scholar]
- 45.Cohen M.A., Evins A.I., Lapadula G., Arko L., Stieg P.E., Bernardo A. The rectus capitis lateralis and the condylar triangle: important landmarks in posterior and lateral approaches to the jugular foramen. J Neurosurg. 2017;127(6):1398–1406. doi: 10.3171/2016.9.JNS16723. [DOI] [PubMed] [Google Scholar]
- 46.Ferrari M., Schreiber A., Mattavelli D., et al. Surgical anatomy of the parapharyngeal space: multiperspective, quantification-base d study. Head Neck. 2019;41(3):642–656. doi: 10.1002/hed.25378. [DOI] [PubMed] [Google Scholar]