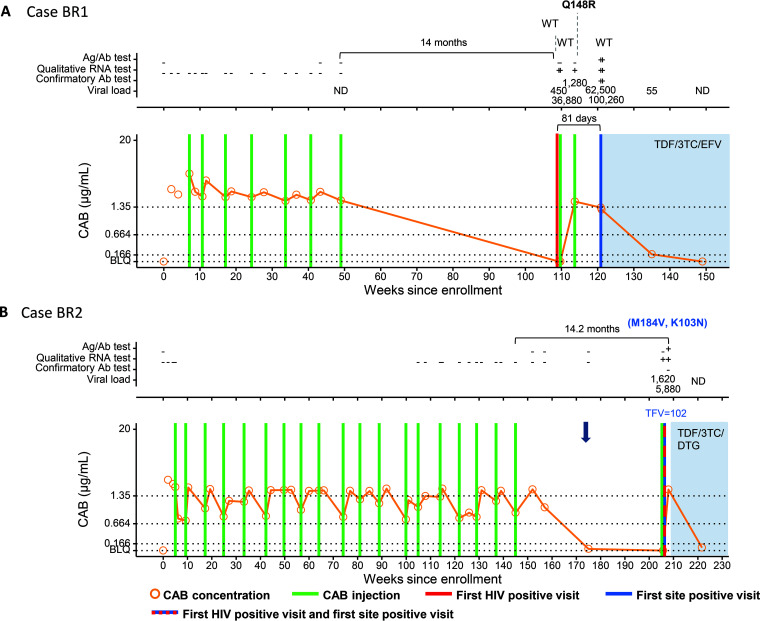
FIG 3.
Summary of key events and laboratory results for participants in the cabotegravir (CAB) study arm of HPTN 083: cases BR1 (A) and BR2 (B). Results from testing performed at study sites are shown in File S2 in the supplemental material. Test results and key study events are shown as a function of the number of weeks from study enrollment based on calendar dates. Results obtained from testing performed at the HPTN Laboratory Center are shown above each graph. Positive and reactive results are indicated with a plus sign; negative and nonreactive results are indicated with a minus sign. IND indicates an indeterminate test result. VL values are shown (number of HIV RNA copies per milliliter). Results from HIV drug resistance testing are shown. All drug resistance mutations are shown for INSTIs; major INSTI mutations are shown in boldface type. Major drug resistance mutations for other drug classes are shown in blue text in parentheses. The brackets at the top of the graph show the number of days between the last injection and the first HIV-positive visit. Lower brackets show the number of days between the first site-positive visit and the first HIV-positive visit for cases where there was a delay in site-detection. The graphs show plasma CAB concentrations and key events. The key at the bottom describes the symbols used in the graphs. Horizontal lines indicate the following concentration cutoffs: 1.33 μg/mL for 8× PA-IC90 (in vitro protein-adjusted 90% CAB inhibitory concentration), 0.664 μg/mL for 4× PA-IC90, and 0.166 μg/mL for 1× PA-IC90. BLQ indicates that the CAB concentration was below the limit of quantification (<0.025 μg/mL). Shaded areas indicate that the participant was on antiretroviral therapy. The blue arrow indicates that tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) was dispensed for preexposure prophylaxis. TFV indicates the concentration of tenofovir in plasma (nanograms per milliliter). 3TC, lamivudine; Ag/Ab, antigen/antibody; DTG, dolutegravir; EFV, efavirenz; ND, not detected; WT, wild type (no RAMs detected).