Abstract
Alzheimer’s disease (AD) is a complex disease that is mediated by numerous factors and manifests in various forms. A systems biology approach to studying AD involves analyses of various body systems, biological scales, environmental elements, and clinical outcomes to understand the genotype to phenotype relationship that potentially drives AD development. Currently, there are many research investigations probing how modifiable and nonmodifiable factors impact AD symptom presentation. This review specifically focuses on how imaging modalities can be integrated into systems biology approaches using model mouse populations to link brain level functional and structural changes to disease onset and progression. Combining imaging and omics data promotes the classification of AD into subtypes and paves the way for precision medicine solutions to prevent and treat AD.
Keywords: Alzheimer’s disease, Imaging, Systems biology, Resilience, Precision medicine
1. Introduction
Alzheimer’s Disease (AD) is a multifaceted neurodegenerative disease that currently has no cure or clinically effective treatments. AD is the most common form of dementia, the 7th leading cause of death globally, and the 6th leading cause in the USA, with more than 6.2 million Americans living with this disease (The Top 10 Causes of Death, 2020). This frequency is estimated to further increase in the US by 2050; however, these estimations may not accurately reflect disease prevalence as many cases likely go undetected due to diagnostic challenges that arise from the highly variable presentation of the disease (Taylor et al., 2017). The lack of consensus about disease manifestation and its typical progression emphasizes the need for improved predictive diagnostic factors. Additionally, the field would benefit from collectively taking a more holistic approach to studying this disease as a series of interacting biological systems and factors rather than examining each involved system in isolation.
The aim of this review is to describe studies that have aided in the understanding and classification of AD using systems biology approaches that exploit imaging methods. We summarize the various factors that influence AD progression, the variable presentation of AD among individuals, and how the field of AD research is evolving to take more systems-level approaches. We focus on studies that successfully link the components of systems biology to clinical outcomes, specifically using imaging data as an intermediate to analyze disease state.
1.1. Types of AD
AD is a debilitating disease that causes progressive decline in cognitive and motor function that significantly reduces one’s quality of life. A definitive diagnosis can only be determined by the postmortem detection of amyloid-beta (Aβ) plaques and tau neurofibrillary tangles (NFT). While these two hallmarks of AD correlate with disease progression, their presence is not completely predictive of AD development as their prevalence varies based on type of AD. Plaque and tangle pathology only account for about 41% of variation in cognitive decline between individuals with AD (2020 Alzheimer’s Disease Facts and Figures, 2020; Boyle et al., 2013).
Traditionally, AD cases are initially classified by genetic inheritance pattern and the age at onset (AAO) of disease symptoms. Thereafter, AD progression is characterized on a continuum based on the extent of cognitive decline and pathological load(s) (Braak et al., 2006; Braak and Braak, 1991; Markesbery, 1997). The two broad categories of AD are early onset AD (EOAD) and sporadic late onset AD (LOAD). EOAD can be subdivided to reflect cases that result from mendelian or nonmendelian inheritance of casual mutations. Mendelian, or familial, AD (FAD), is characterized by the inheritance of highly penetrant, autosomal dominant causal mutations in the genes APP, PSEN1, and PSEN2 (Mendez, 2017; Tanzi, 2012). These mutations only account for a small percentage of FAD cases, and variation in age of onset and severity of symptoms exists among individuals, suggesting that additional genetic and environmental factors modify disease pathogenesis and clinical manifestation (Ryman et al., 2014). Nonmendelian, or non-familial, EOAD is classified by the aggressive onset of cognitive symptoms before the age of 65; however, individuals with this form of AD develop symptoms sporadically and have inconsistent inheritance patterns (Joshi et al., 2012; Reitz et al., 2020).
LOAD is the most common form of AD, occurring in individuals 65 years and older, with highly variable presentation of symptoms, which also vary in severity. Age is the greatest risk factor for LOAD, but research suggests that there are also additional causal genetic and environmental factors. According to twin and family studies, LOAD is approximately 58% to 79% heritable and gene variants in APOE and TREM2 are established LOAD risk factors (Belloy et al., 2019; Corder et al., 1993; Gatz et al., 2006; Pedersen et al., 2004; Räihä et al., 1996; Roses, 1996; Strittmatter et al., 1993a; Strittmatter et al., 1993b). To date, more than 30 genetic risk variants and susceptibility loci have been identified by genome-wide association studies (GWAS) or phenome-wide association studies (PheWAS), including CLU, BIN1, ABCA7, and SORL1 (Andrews et al., 2020; Backman et al., 2021; Bellenguez et al., 2020; Kunkle et al., 2019; Lambert et al., 2013; Pimenova et al., 2018; Wang et al., 2016b; Wightman et al., 2021; Zhao et al., 2019). Individually, each AD-associated locus or gene variant has a relatively small effect on the likelihood of developing AD. AD risk increases with each genetic variant inherited, which overall has an additive effect on AD severity; this is referred to as polygenic risk. The compilation of genes identified using GWAS allows for the assignment of polygenetic risk scores which can aid in predicting risk or disease progression; however, additional factors such as sex and environment also need to be taken into consideration to gain a comprehensive understanding of the disease and how it manifests in individuals (Dunn et al., 2019).
Recent studies have begun to reprioritize GWAS hits by integrating multiscale data collected from relevant brain regions of interest (e.g. hippocampus) to generate network-based functional prediction methods and gene-related imaging biomarkers (e.g. brain atrophy) (Elliott et al., 2018; Knutson et al., 2020; Meng et al., 2020; Shen et al., 2010; Wachinger et al., 2018; Xu et al., 2017). Additionally, GWAS methods and imaging data have been aggregated to identify loci associated with image-derived phenotypes (Cruchaga et al., 2013; Elsheikh et al., 2020; Furney et al., 2011; Grasby et al., 2020; Hofer et al., 2020; Li et al., 2017; Matoba and Stein, 2021; Meda et al., 2012; Nativio et al., 2020; Ramanan et al., 2015; Smith et al., 2021). Ultimately, this method of combining omics and imaging data to link changes in gene expression, the biological pathways associated with those genes, and functional and structural changes in the brain, may allow researchers to further assess both EOAD and LOAD and potentially narrow down these disease classifications in subtypes.
1.2. Sex differences
Females have a higher prevalence of AD and experience more severe cognitive and noncognitive symptoms than men (2019 Alzheimer’s Disease Facts and Figures, 2019). Previously, this unequal distribution of cases was attributed to the longer average lifespan of females, but in recent years more specific evidence linking sex and AD progression has been identified (Mielke et al., 2014). Among those with FAD, global amyloid load and greater tau deposition in the frontal, inferior parietal, and temporal lobes was higher in females (Groh et al., 2020; Oveisgharan et al., 2018). Interestingly, sex differences in AD development varies based on pathology load. Both males and females with low pathology load have similar risks of developing AD, whereas in individuals with moderate to high levels of pathology, disease risk is greater in females (Barnes et al., 2005). Females diagnosed with AD also experience a faster progression of hippocampal atrophy compared to males (Ardekani et al., 2016). With increased numbers of study participants to enhance statistical power, as well as computational resources and large collaborative research teams, sex-stratified GWAS have led to identification of sex-specific genetic factors that drive pathology and AD progression (Deming et al., 2018; Nazarian et al., 2019; Prokopenko et al., 2020). Expression quantitative trait loci (eQTL) mapping was performed on putative sex-specific GWAS loci to identify candidate genes that were associated with a range of AD markers for each sex. Using this method, a single nucleotide polymorphism (SNP) of the candidate locus MAPT was positively associated with NFT specifically in males (Dumitrescu et al., 2019). Until recently, sex was typically controlled or adjusted for as a demographic factor in most human studies, but as experiments continue to highlight the importance of sex-specific differences in AD risk and development, it is apparent that sex needs to be more thoroughly studied in a controlled manner, while taking environmental exposures into consideration, via longitudinal investigations.
1.3. Environmental control
The relationship between AD and environmental factors has increasingly become a research topic of interest as correlations and comorbidities between AD and modifiable behaviors have been uncovered. Strikingly, recent meta-analyses have found that up to 40% of dementia and AD cases may be attributed to controllable environmental factors throughout a person’s life (Barnes and Yaffe, 2011; Livingston et al., 2020; Livingston et al., 2017). Links between the interrelated health factors or AD “exposomes” including diet, exercise, chronic stress, other environmental exposures, and AD development have been acknowledged (Biessels et al., 2006; Cui et al., 2018; De la Rosa et al., 2020; Finch and Kulminski, 2019; McGrattan et al., 2019; Wild, 2012; Yang and Song, 2013). Environmental considerations also include investigating epigenetics and gene by environment (GxE) interactions by implementing GWAS to better understand genetic regulators of environmental effects and provide novel insights and targets for precision medicine solutions (Dhana et al., 2020; Eid et al., 2019; Hohman and Kaczorowski, 2020). The list of modifiable environmental factors that potentially impact AD progression continues to increase as research techniques and technology evolve to better survey large populations. Each of these factors and many others play a synergistic role and likely interact with genes to modify expression resulting in a certain phenotype. These factors and their effects are conditional in their role in AD development and progression (Chouliaras et al., 2010). For instance, aspects of weight control have been subjected to evaluation as certain diets and exercise regimes have proven to be beneficial to long term health and reduced disease incidence in later life. Reduced weight is often seen as a biomarker for AD that can occur even a decade before the onset of cognitive symptoms (Barrett-Connor et al., 1996; Buchman et al., 2005; Gillette-Guyonnet et al., 2000; Johnson et al., 2006; Wolf-Klein et al., 1992). When relating body mass index (BMI) and polygenetic risk scores calculated using all SNPs from a recent AD GWAS in humans, lower BMI and higher polygenic risk score significantly predicted conversion to AD (Moody et al., 2021). Conversely, early and mid-life increased weight and obesity, including that linked to high-fat/high sugar Western diet consumption is associated with increased risk of AD and dementia (Naderali et al., 2009; Profenno et al., 2010; Tabassum et al., 2020). Overall, studying environmental effects on AD in human populations is extremely challenging due to lack of experimental control and wide amount of environmental variation humans are exposed to. This is further exacerbated because most studies rely on participant self-reporting and these results are often inaccurate and inconsistent (Cherbuin and Anstey, 2012; Otaegui-Arrazola et al., 2014; Rueda et al., 2015; Singh et al., 2014; Yusufov et al., 2017). When these inconsistencies are paired with the overwhelming amount of genetic diversity among humans, attempts to elucidate GxE interactions that influence AD are experimentally difficult.
1.4. Opportunity to complete longitudinal studies
While sex and environmental factors contribute to the development and progression of AD, age is the greatest non-modifiable risk factor and the primary driver of developing AD. Disease risk dramatically increases after 60 years of age, but AD is not a normal aspect of aging, and not all individuals that exhibit hallmark AD pathology or symptoms develop AD (Hebert et al., 2013; Sonnen et al., 2011; Toepper, 2017). The definition of AD stages has evolved and become more dynamic as researchers have determined that disease development varies between individuals. Initially, the stages of AD were defined at the autopsy of individuals that showed clinical signs of AD, like severe memory impairment, in life. Postmortem analysis of AD stages were ultimately based on the regional distribution, type, and density of brain pathology (Braak and Braak, 1991). Recently, preclinical and presymptomatic stages of AD were identified based on pathology in the post-mortem analysis of brains of cognitively unimpaired people. This suggests that disease onset can be defined differently depending on the evaluation of brain pathology versus clinical symptoms (Dubois et al., 2016; Hubbard et al., 1990; Sandberg et al., 2001; Villemagne et al., 2011). The discovery that AD-related changes in the brain and pathology accumulation can begin potentially decades before the onset of clinical symptoms revealed potential confounds in previous AD cross-sectional studies that only analyzed individuals with MCI and AD versus “cognitively healthy control” subjects, as their control groups could have included pre-symptomatic individuals with AD pathology (Aisen et al., 2017; Bennett et al., 2006; Driscoll and Troncoso, 2011; O’Brien et al., 2009; Price et al., 2009). In addition to identifying asymptomatic and prodromal phases of AD, recognition of hallmark AD pathology in cognitively intact individuals has also led to the classification of resilience and susceptibility to AD-related decline (Aiello Bowles et al., 2019; Driscoll and Troncoso, 2011; Dumitrescu et al., 2020; Hampel et al., 2019a; Hohman et al., 2016; Negash et al., 2013; Neuner et al., 2017b; Stern et al., 2020; Walker and Herskowitz, 2020). Longitudinal efforts to identify biomarkers and endophenotypes that allow for refined stage assessment are more crucial than ever as preclinical stages at which hallmark symptoms are not detectable may be an opportune period to engage in disease slowing or prevention measures. Additionally, understanding what factors shield resilient individuals versus those that cause others to be severely susceptible to AD development may provide key insight for treatment advancement (Seto et al., 2021). Longitudinal studies allow for the evaluation of AD as a continuum, but most of these studies only follow up with patients for an average of 1–2.5 years with limited repeated measures (Lawrence et al., 2017). Furthermore, only a few longitudinal human studies and designated aging cohorts such as the Religious Order Study, Mount Sinai Brain Bank study, or Rush Memory and Aging Project have the capacity to comprehensively assess disease progression (Bennett et al., 2018; De Jager et al., 2018; Wang et al., 2018). Current human biomarkers measured longitudinally lack the sensitivity to identify early disease stages and disease subtypes (Cummings, 2019). Ultimately, there is a need for model systems to better investigate the early stages of AD, AD causation, and to take a higher resolution look at changes in brain structure that occur with age and disease progression, especially during the stage when AD is clinically silent, and no overt symptoms are detected.
1.5. The call for mouse models of AD
Mouse models of AD offer the opportunity to study the disease longitudinally and in a more controlled manner to gain a better understanding how it manifests and progresses in humans. Mouse models are particularly advantageous because they can provide replicable genomes in controlled environments across relatively short lifespans, which can be implemented to address gaps in human research. Although many models are pathology-centric, there are currently over 205 existing AD mouse models that vary in their presentation of plaques, tangles, neuronal loss, gliosis, and synaptic dysfunction (Research Models: Alzheimer’s Disease, 2021). These models mostly consist of transgenic, knock-in (KI), or out (KO) modifications of single genes or a combination of genes associated with human AD, including APP, PSEN1/2, APOE, Trem2, BACE1, BACE2, MAPT and other GWAS-identified genes on various background strains (Drummond and Wisniewski, 2017). These models display AD-related phenotypes that can be accurately assessed and associated with disease progression (Götz et al., 2018; Granic et al., 2010; Keene et al., 2016; Romberg et al., 2013).
Recapitulating human AD (particularly LOAD) in mouse models has proven difficult; therefore, choosing the appropriate AD model mouse population is crucial since many models selectively display different aspects of the disease and mouse findings have not translated well to humans (Cao et al., 2018; Cummings et al., 2014; Franco and Cedazo-Minguez, 2014; Jankowsky and Zheng, 2017; King, 2018). A reason for this lack of translatability is that most traditional mouse models of AD are made using genetically identical mice and lack the genetic diversity present in humans (Moore et al., 2020; Onos et al., 2016). While traditional mouse models, which were needed, timely, and useful for their era, were a great starting point for using model systems to study AD, it is now apparent that they are not the most translationally relevant models available and that genetic diversity is crucial for both the development of models and AD mouse research moving forward.
1.6. Translatable mouse models
To combat the limitations of traditional mouse models, mouse models with diverse genetic backgrounds have recently been generated and utilized to study AD in a more translational manner (Neff, 2019; Neuner et al., 2019a; Neuner et al., 2017a; Neuner et al., 2020; O’Connell et al., 2019; Onos et al., 2019; Yang et al., 2021). For example, genetic diversity can be added to standard AD mouse models with the incorporation of BXD recombinant inbred strains. The BXD panel is the product of independent advanced intercrosses between C57BL/6 J (B6) and DBA/2 J (D2) progenitor strains (Peirce et al., 2004). Application of the BXD panel is conducive to systems biology approaches, as the panel has a genetically defined diverse background that can be easily manipulated in a reproducible manner. The BXD family segregates at over five million common genetic variants and more than 140 strains are currently available (Wang et al., 2016b). These additional BXD strains offer greater mapping power and the ability to refine mapping precision (Ashbrook et al., 2021). The BXD population has been highly characterized in a variety of studies, creating a wealth of phenotyping and omics data (Studies Involving BXD RI Panel, 2021). The BXD population was demonstrated to be a valuable resource for creating the first mouse model that better recapitulates the complex heterogeneity of genetic, molecular and cognitive features of human cognitive aging and AD (Neuner et al., 2016; Neuner et al., 2019a; Neuner et al., 2017a). The AD-BXD population was generated by crossing the commonly used B6–5XFAD AD mouse model with strains from the BXD panel. The AD-BXD panel offers all of the advantages of the BXD population in an AD mouse model, and, importantly, this model mouse panel exhibits a range in age at onset and variation in AD symptom severity that is comparable to LOAD in human populations (Neuner et al., 2019b; Neuner et al., 2017a; Ryman et al., 2014). This panel also exhibits a high degree of genetic and transcriptomic overlap with human LOAD (Heuer et al., 2020; Lambert et al., 2013; Neuner et al., 2019a; Neuner et al., 2017a; Wan et al., 2020). Ultimately, genetically diverse panels like the AD-BXD that recapitulate multiple facets of AD offer the scientific community a more applicable model system to study the genetic mechanisms that modify the onset and progression of AD across a population.
1.7. Use of systems biology to better understand the complexity of AD
Following the advent of the amyloid beta cascade hypothesis as a proposed cause of AD, numerous clinical trials targeted the reduction and prevention of amyloid plaques in an attempt to lessen the symptoms and progression of AD (Hardy and Selkoe, 2002; Hardy and Higgins, 1992; Lemere and Masliah, 2010; Reitz, 2012; Schneider et al., 2014). None of these trials successfully alleviated pathology progression, neurodegeneration, or major long-term symptoms, therefore forcing the research community to acknowledge the immense complexity of AD (2020 Alzheimer’s Disease Facts and Figures, 2020; Cao et al., 2018; Chen et al., 2017; Langley, 2014). Since this realization, AD researchers more commonly utilize systems biology approaches to better understand interactions between various systems in the human body and how they impact, and are impacted by, AD (Alberghina and Colangelo, 2006; Castrillo et al., 2018; Lista et al., 2016; Rosario et al., 2020). The fact that we observe similar disease phenotypes despite differences in genetic modulators (ex: between FAD and LOAD) suggests that the different causes of disease are not unrelated but are rather likely due to dysregulation of similar biological networks. Systems biology is a field of study built on the organization of sub fields responsible for complex behaviors and outcomes, including identifying the links between genes and behavior according to the net interactions of varying components (Liu, 2005). Modern systems biology involves interdisciplinary, data-driven approaches with a greater focus on untangling complex interactions between genetic, epigenetic, physiological, and environmental factors at multiple system levels within an organism. Recent advancements in biotechnology have made this approach more feasible and enable genome-wide and multi-omics studies to be conducted with multiple disease-mediated factors (Heuer et al., 2020; Lam et al., 2020). Systems biology puts a greater emphasis on connection, integration and modularity of genes and pathways rather than single causal gene predictions. This approach is crucial for the study of complex diseases like AD because their cures require multifaceted treatments tested in diverse and translatable models. Development of such a treatment requires the implementation and integration of transcriptomics, proteomics, metabolomics, genomics, epigenomics, lipidomics, and/or micro-biomics to gain a wholistic understanding of complex systems across representative populations (Fig. 1) (Ahn et al., 2006; Ehrenberg et al., 2003; Hiesinger and Hassan, 2005; Kirschner, 2005; Kitano, 2002b; Liu, 2005; Weston and Hood, 2004). Ultimately, the harmonization of multiple data types across various body systems will provide a better method of surveying the many components involved in the development and progression of AD.
Fig. 1.
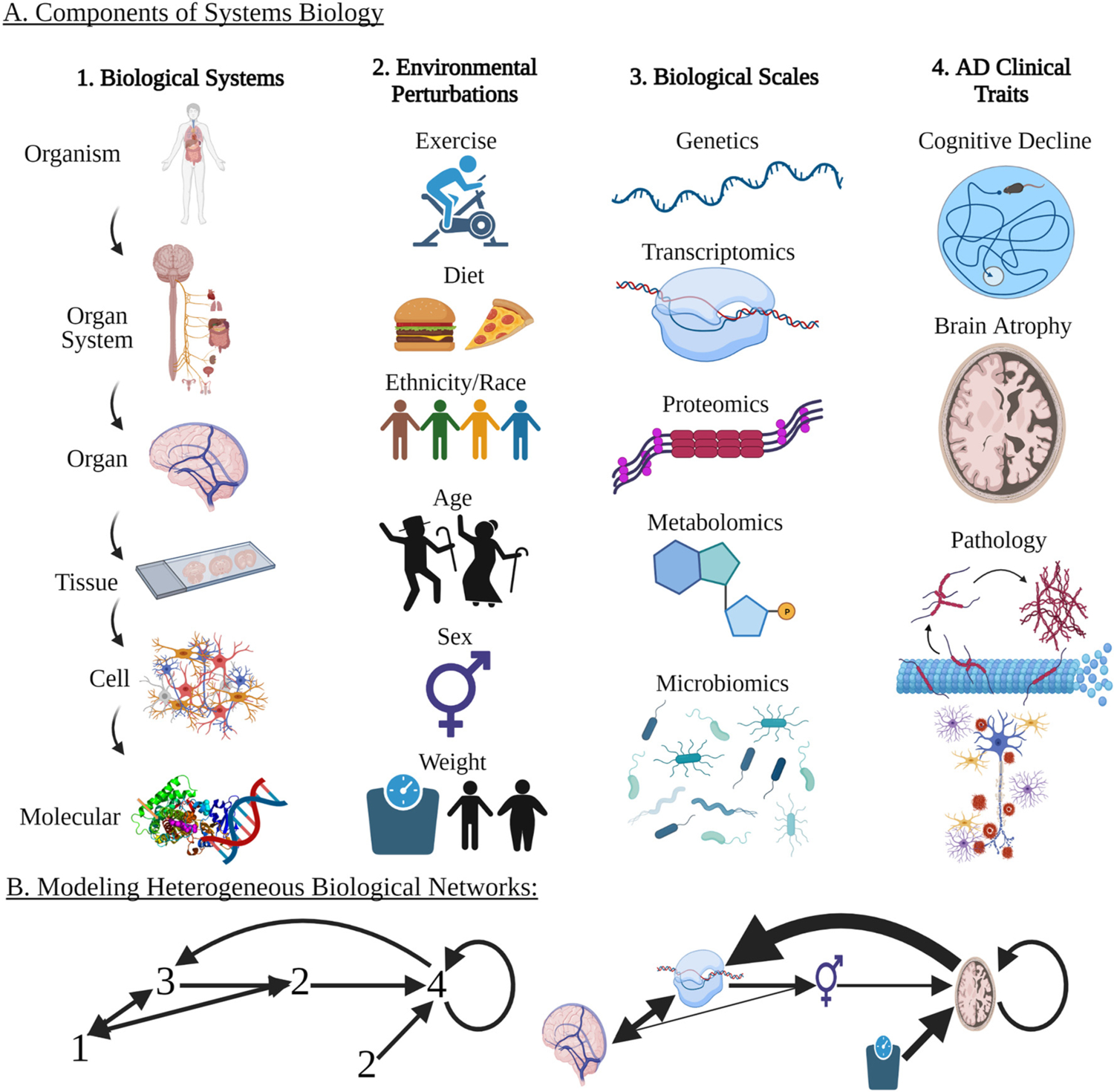
Analyzing the Interacting Components of System Biology in the Study of Alzheimer’s Disease (A) The study of systems biology and the discovery of genotype to phenotype relationships involves the interaction of multiple levels: 1. biological systems, 2. environmental perturbations, 3. biological scales, and 4. clinical traits. (B) Because AD is a complex disease, modeling of biological networks is required to test and discover the relationship between factors and mechanisms. Combinations of single or multiple factors from each biological and environmental scale (A:1–4) should be included in models to determine the correlation between data types and resulting clinical outcomes. (B) shows a hypothetical model of how the factors from (A) could interact. Each factor can impact others with varying weights of influence indicated by the width of the arrows. (Created with BioRender.com).
Expanding beyond correlational analyses, systems biology has also benefitted from the advent of causal inference methods in establishing links between genotype and phenotypes, and all systems in between (Haas et al., 2016; Shen et al., 2020). Adopting this approach to studying AD, national and international human and mouse focused consortia were launched to integrate data types to gain a better understanding of the brain and the changes that occur in response to onset of AD. Initiatives by The Alzheimer’s Association, Human Brain Project, and Foundation for the National Institutes of Health Biomarkers Consortium have all taken steps to implement systems biology approaches to studying AD. The National Institute on Aging’s AD Translational Research also established programs including Accelerating Medicines Partnership- AD (AMP-AD), Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease (M2OVE-AD), Translational Center for Model Development and Evaluation for Late Onset Alzheimer’s Disease (MODEL-AD), Cognitive Resilience to Alzheimer’s Disease (Resilience-AD), and Neuropsychiatric Symptoms in Alzheimer’s Disease (Psych-AD), which are dedicated to uncovering the multifaceted roots of AD. Furthermore, there are initiatives dedicated to including specific methods such as the Alzheimer’s Disease.
2. AD diagnosis: from the lab to the clinic
2.1. Imaging modalities to assess AD
With advancements in technology, imaging modalities have recently become a highly effective method for identifying and monitoring age-and AD-related structural and functional changes in the brain. Modern forms of microscopy implemented in mouse models allows for better spatial and temporal resolution images than ever before. Cross sectional whole 2D and 3D brain mapping at different disease stages and ages can now be used to identify regional vulnerability to pathology or changes in specific cell types, especially in deeper brain regions difficult to access in vivo (Chen et al., 2018a; Gail Canter et al., 2019; Lichtenegger et al., 2018; Munoz-Castaneda et al., 2021; Whitesell et al., 2019). These techniques can also be applied in transgenic mice or those injected with specific tracers, such as those used to label active neurons during a memory task, to establish connections between regional activation and behavior (Roy et al., 2019; Vetere et al., 2017). While the application of imaging modalities can be readily applied in mouse models, currently these methods have been most thoroughly investigated in humans.
Improvements in in vivo imaging resolution and accessibility now allow for human AD diagnoses prior to pathology and atrophy detection at death. The most commonly utilized methods include the minimally-invasive magnetic resonance imaging (MRI) and positron emission tomography (PET), which give researchers and medical teams a better look at the active brain to then make a diagnosis and assess disease state (Marcus et al., 2014; Márquez and Yassa, 2019; Scheltens, 2009; Smith, 2002). Various imaging approaches (outlined in Table 1) allow for the detection of neural connectivity deficits, the presence and progression of pathology, tissue atrophy, and even metabolic measures. These measures can then aid in the discovery of brain regions vulnerable to specific measures collected during each imaging processes (Johnson et al., 2012; Pini et al., 2016; Reiman and Jagust, 2012; Teipel et al., 2015; Young et al., 2020). Since many features of AD can be detected non-invasively, imaging can be readily used to increases the accuracy of clinical assessments and monitored longitudinally (Jack et al., 2013; Jack Jr. et al., 2010; Jagust et al., 2006; Karow et al., 2010; Oishi et al., 2011; Ota et al., 2015; Zhang et al., 2011). Identification of predictive AD biomarkers and patterns in structural and functional changes can be used as endophenotypes to relate to other aspects of systems biology to address the biological mechanisms driving AD progression. This integration of imaging and omics data collected with systems biology approaches, or neuroimaging-omics, is an emerging field dedicated to characterizing genetic, biological, and phenotypic clusters which can then be used to develop methods for detecting, treating, or possibly preventing disease development with early intervention (Hampel et al., 2021; Mroczek et al., 2021; Richiardi et al., 2015). Many neuroimaging-omics studies employ machine learning frameworks to multi-modal data to predict potential AD risk in MCI and pre-symptomatic patients (Basaia et al., 2019; Khanna et al., 2018; Scelsi et al., 2018). Neuroimaging-omics has the potential to untangle genetic mutations, gene expression patterns, and protein-protein interactions and determine how they are linked to large-scale structural and functional network deficits and disease manifestation.
Table 1.
Neuroimaging in AD: modalities and typical findings.
| Imaging modality | Brain measurement | Measured changes with relation to AD | Citations |
|---|---|---|---|
| F-fluorodeoxyglucose (FDG) PET |
|
|
(Brown et al., 2014; De Santi et al., 2001; Foster et al., 1983; Foster et al., 2007; Hoffman et al., 2000; Marcus et al., 2014; Mosconi, 2005; Silverman et al., 2001) |
| Amyloid PET |
|
|
(Agdeppa et al., 2001; Cselényi et al., 2012; Kudo et al., 2007; Landau et al., 2014; Ossenkoppele et al., 2012; Rinne et al., 2012; Rowe et al., 2008; Verhoeff et al., 2004; Wong et al., 2010) |
| Tau PET |
|
|
(Brier et al., 2016; Cho et al., 2016; Jack Jr. et al., 2018; Leuzy et al., 2019; Maass et al., 2017; Mueller et al., 2020; Scholl et al., 2019; Wang et al., 2016a) |
| Synaptic vesicle glycoprotein 2A (SV2A) PET |
|
|
(Bastin et al., 2020; Cai et al., 2019; Chen et al., 2018b; Finnema et al., 2018; Mecca et al., 2020; O’Dell et al., 2021) |
| Translocator Protein-18 kDa (TSPO) PET |
|
|
(Edison et al., 2008; Kreisl et al., 2016; Kreisl et al., 2013; Lagarde et al., 2018; Mirzaei et al., 2016; Tournier et al., 2020; Yasuno et al., 2008) |
| Structural MRI |
|
|
(Cardenas et al., 2011; Hua et al., 2008; Plant et al., 2010; Ridha et al., 2008; Spulber et al., 2013; Vemuri and Jack, 2010) |
| Functional MRI- resting state or tasks dependent |
|
|
(Bookheimer et al., 2000; Dickerson and Sperling, 2008; Logothetis et al., 2001; Machulda et al., 2003; Ogawa et al., 1990; Rombouts et al., 2000; Sperling et al., 2010; Vemuri et al., 2012) |
| Diffusion Tensor Imaging (DTI) |
|
|
(Alexander et al., 2007; Colon-Perez et al., 2019; Febo et al., 2020; Harrison et al., 2020; Mayo et al., 2019; Ofori et al., 2019; Ouyang et al., 2015; Sahara et al., 2014) |
2.2. Technology’s contribution to enhancing the imaging field and AD experiments
Disease characterization and identification of AD biomarkers with imaging analyses have significantly progressed due with advances in modern technology. Methods to accommodate the large datasets required to power systems biology experiments are being streamlined to reduce the significant subjectivity and time commitment previously required to obtain and interpret results from imaging studies. Using automated pipelines that incorporate standard brain atlases, like the Allen Brain Atlas Common Coordinate Frame, comprehensive connectivity maps are being developed to better understand mammalian brain circuitry (Denk et al., 2012; Wang et al., 2020). The Mouse Brain Architecture Project, Allen Mouse Brain Connectivity Atlas project, and Mouse Connectome Project each have taken on the challenge of systematically mapping the spatial profiles and the connectivity of neuronal populations throughout the brain (Bohland et al., 2009; Furth et al., 2018; Helmstaedter and Mitra, 2012; Mitra, 2014; Oh et al., 2014; Osten and Margrie, 2013). Through these investigations and others, a myriad of image analysis pipelines been created to investigate links between regional brain activity, gene expression, and behavior (Feng et al., 2015; Freeman et al., 2014; Ji et al., 2014; Ng et al., 2009; Renier et al., 2016). Computational biologists are also creating these workflows to increase reproducibility, make machine learning and automated imaging processing methods more accessible to biologists, and allow high-throughput processing across the brain. Semi-automatic registration methods with all parameters shared with the scientific community encourage non-experts to analyze high resolution MRI, DTI, histology, and two-photon tomography results (Anderson et al., 2019; Budin et al., 2013; Esteban et al., 2019; Furth et al., 2018; Liu et al., 2020; Niedworok et al., 2016; Pagani et al., 2016; Pallast et al., 2019; von Chamier et al., 2021; Winnubst et al., 2019; Yates et al., 2019). The recent combination of neuroimaging, computer-aided diagnosis techniques, and machine learning methods (e.g. linear discriminant, logistic regression, random forest, and neural networks analyses) have allowed researchers and clinicians alike to establish data-driven AD classification standards (Basheera and Sai Ram, 2019; Dimitriadis et al., 2018; Leandrou et al., 2018; Liu et al., 2019; Wen et al., 2020). Integrating multi-omics data is also increasingly more feasible and approachable for biologists with the development and improvement of omics technologies aimed at aiding the combination and analysis of multiple data types (outlined in Table 2). The availability and application of current tools have also been thoroughly reviewed (Adil et al., 2021; Graw et al., 2021; Huang et al., 2017; Krassowski et al., 2020; Ma et al., 2020; Misra et al., 2018; Nicora et al., 2020; Subramanian et al., 2020; Worheide et al., 2021). One noted drawback of many of the mentioned tools in these reviews is that they do not necessarily have the capacity to incorporate imaging or behavioral phenotyping data in addition to the varying omics data.
Table 2.
Tools for multi-omics integration, viewing, and analysis.Can Table 2 and 3 be widen and displayed as one data frame like Table 1 is formatted in the PDF? Each table is currently split in the proof.
| Tools to view and/or analyze multi-omics datasets | Purpose of tool | Platform | Citation |
|---|---|---|---|
| Multi-Omics Factor Analysis (MOFA) |
|
Python (mofapy2) and R (MOFA2) | (Argelagu et al., 2018) |
| MixOmics |
|
R Package | (Rohart et al., 2017b) |
| MixOmics: Multivariate INTegrative method (MINT) |
|
R package | (Rohart et al., 2017a) |
| MixOmics: Data Integration Analysis for Biomarker discovery using Latent cOmponents (DIABLO) |
|
R package | (Singh et al., 2019) |
| Similarity network fusion (SNF) |
|
R and Matlab Code | (Wang et al., 2014) |
| Paintomics |
|
Web-based | (Hernandez-de-Diego et al., 2018) |
| 3Omics |
|
Web-based | (Kuo et al., 2013) |
| JIVE |
|
R Package | (Lock et al., 2013) |
| MiBiOmics |
|
Web-based | (Zoppi et al., 2021) |
| Multi-Omics Graph cOnvolutional NETworks (MOGONET) |
|
Python Package | (Wang et al., 2021) |
| Survival Analysis Learning with Multi-Omics Neural Networks (SALMON) |
|
Python Package | (Huang et al., 2019) |
| NEMO (NEighborhood based Multi-Omics clustering) |
|
R Package | (Rappoport and Shamir, 2019) |
| Galaxy |
|
Web-based | (Boekel et al., 2015) |
| Argonaut |
|
Web-based | (Brademan et al., 2020) |
| Alzheimer’s Disease Alternative Splicing-Viewer (ADAS) |
|
Web-based | (Han et al., 2021) |
| Genome-wide Positioning Systems platform for Alzheimer’s Drug Discovery (AlzGPS) |
|
Web-based | (Zhou et al., 2021) |
Moreover, systematic machine-learning assisted approaches to imaging are no longer exclusively restricted to post-processing analyses but also to assist in experimental parameter design to enhance microscopy techniques and output. This field of “smart microscopy” integrates feedback from the microscope to adjust computer assisted imaging algorithms to optimize sample coverage, extend the field of view, or improve spatial resolution and signal strength (Durand et al., 2018; He and Huisken, 2020; Mahecic et al., 2020; Royer et al., 2016). Researchers are now equipped with the ability to map regional behavior-induced brain activity, which can prove to be highly valuable in determining which regions, cell types, and circuits are most heavily affected by AD and in response to certain tasks assessing clinical symptoms.
3. Incorporating imaging outcomes in mouse systems biology studies to predict cognitive outcomes and AD progression
Imaging approaches have proven to be an invaluable resource to the field of AD research in terms of assessing changes in neuroanatomy and neural connections; however, attempts to link imaging measures and multi-omics data to phenotypic disruptions associated with AD in model systems remain scarce. Furthermore, many of these studies are not sufficiently powered with the number of mice needed for the application of systems biology approaches. Although current approaches for assessing the brain using imaging methods do not allow for the discovery of the mechanisms and interacting relationships driving these changes, efforts to link imaging measures to cognitive outcomes are underway. Imaging modalities used to visually assess disease progression provides researchers with an intermediate to model the relationship between structure and function in model mice.
Depending on the study of interest, imaging data is independently compared to or directly correlated with cognitive functioning within cohorts of model mice to assess functional or connectivity properties in relation to level of AD-related decline. MRI studies using a variety of model mouse lines have described changes in regional neuronal activity, differences in volume, and structural integrity to identify vulnerable brain regions. For example, manganese enhanced MRI (MEMRI) can be used to map complex brain circuits involved in spatial memory. Decrease in MEMRI signal after Morris water maze (MWM) testing corroborates the reduced neuronal activity in memory circuits typically seen in old AD mice (Badea et al., 2019). Similarly, identification of regional atrophy in relation to behavioral outcomes can also be explored. Among several types of AD model populations, atrophy at the whole brain level, as well as memory associated areas like the hippocampus, entorhinal cortex, amygdala, and temporal association cortex has been observed in mice with reduced spatial memory (Liang et al., 2017; Tang et al., 2016). Depending on the mouse line used, these changes in brain structure and connectivity can be observed as early as 2 months of age, preceding the onset of amyloid deposition and severe cognitive decline (Badea et al., 2010; Falangola et al., 2007). Studies that validate changes in MRI volume with histology provide further evidence that MRI outcomes that identify regional atrophy correspond to reductions in neuron counts and poor spatial memory performance (Badea et al., 2019). However, currently there is a lack of consensus regarding regional atrophy or enlargement in mice and how this difference relates to reduced cognitive performance on memory tasks and certain ages (Badhwar et al., 2013; Maheswaran et al., 2009).
Brain metabolism has also been related to cognitive performance using fluorodeoxyglucose (FDG) PET. Correlation analyses have showed that hippocampal standardized uptake values were significantly correlated with MWM parameters at the symptomatic-AD stage (Li et al., 2016). Aged Tg4-42 transgenic animals with compromised spatial memory also display neuron loss, regional volume decreases, and hypometabolism – as measured by reduced tracer uptake, in the hippocampus, forebrain, hypothalamus, amygdala and midbrain (Bouter et al., 2018). Likewise, neuroinflammatory response supported by histology shows significant effects of age and genotype on translocator protein (TPSO) tracer uptake in the hippocampus and cortex exist in APPswe × PS1Δe9 transgenic mice, but their working memory performance greatly varied with age (Chaney et al., 2018). Serial PET measures of TSPO and amyloid with terminal spatial memory assessment in PS2APP model mice, followed by immunohistochemical analyses of microglia, amyloid, and synaptic density revealed that high microglial activation at the onset of amyloidosis (8 m of age) predicts better cognitive performance in PS2APP mice at follow-up 5 months later (13 m of age), when amyloid pathology is extensive. Highest TSPO PET signal was found in areas associated with spatial learning and negatively correlated with Iba1 immunostaining (Focke et al., 2019). Conducting multi-modal analyses help in defining a more precise relationship between cognitive outcomes and morphological changes of the brain. Moreover, in vivo 2-photon calcium imaging evaluation of APP23xPS45 mice has shown neuronal hyperactivity near Aβ plaques. With this information, a correlation was observed between the formation of amyloid plaques, the appearance of hyperactive neurons, and the age-related impairment of the spatial learning capability (Busche et al., 2008). Further studies investigating neuronal hyperactivity revealed that the function of hippocampal neurons is altered long before that of cortical neurons (Busche et al., 2012).
Moving beyond comparisons between imaging measures and behavior, the field is turning towards probing neuroimaging-omics interactions in mice (Liu and Liu, 2011). For example, genetic mapping of phenotypes derived from imaging data using the BXD panel revealed significant quantitative trait loci associated with traits such as hippocampal volume (Ashbrook et al., 2014). Cellular and pathology loads calculated using brain-wide immunohistochemistry can also be integrated with bulk RNA sequencing data to evaluate associations between regional cell counts, gene expression, and biological, molecular, or cellular pathways (Gurdon et al., 2020). Moreover, spatial transcriptomics offers the ability to survey regional transcriptional changes in mice. Using this method, molecular changes occurring in cells in the vicinity of amyloid plaques can be investigated by characterizing gene co-expression networks that appeared to be highly responsive to Aβ deposition. The plaque-induced genes (PIGs) are a response in multiple cell types across the brain and are implicated to involve the complement system, oxidative stress, lysosomes, and inflammation, all of which are more prominent in the later phases of the disease (Chen et al., 2020). Furthermore, regions vulnerable to AD-related changes can be honed in on to uncover molecular targets, such as those that may contribute to early hippocampal synaptic deficits and olfactory dysfunction in AD mice (Navarro et al., 2020). Ultimately, these studies and many more incorporate select aspects of systems biology and imaging methods to tie together genotype and phenotype; however, many studies that employ imaging techniques are lacking complementary omics and behavioral data to achieve a comprehensive analysis of brain changes with AD.
4. Refining AD diagnosis with the establishment of subtypes
There is a novel opportunity to more optimally segregate AD into subtypes by combining in vivo depictions of AD progression (collected via imaging) with omics data. By refining AD diagnoses beyond typical, resilient, or susceptible, researchers will be able to better understand the heterogeneity of AD symptoms, manifestation, and causal influences, which will be crucial for executing precision medicine approaches and developing successful treatments for AD.
Noticing that not all human AD cases neatly follow Braak staging, researchers have begun to classify subtypes of AD based on the detection of regional pathology in conjunction with clinical data. Early approaches to tackle this discrepancy evaluated cases that had severe Braak scores and subdivided them by NFT density and location. Three postmortem classifications of typical, hippocampal sparing, or limbic predominant AD were derived and further characterized in terms of prevalence in the experimental population, age demographics, and rate of cognitive decline exhibited within each subtype (Murray et al., 2011). Building on this concept, tau-PET in combination with demographic data, clinical outcome measures, and APOE e4 frequency was used to refine these subtypes into pathology driven region-specific subtypes prior to death (Armstrong and Wood, 1994; Charil et al., 2019; Ossenkoppele et al., 2020; Vogel et al., 2021; Whitwell et al., 2018). Recent approaches that further evaluated these subtypes found that there is largely a consensus between subtyping based on tau-PET and regional atrophy measured using structural MRI methods (Kolanko and Malhotra, 2018; Park et al., 2017; Ten Kate et al., 2018). Differential patterns of brain atrophy revealed general and reproducible subtypes of AD, including typical, limbic-predominant, hippocampal-sparing, mild atrophy, and no atrophy (Byun et al., 2015; Ferreira et al., 2017; Karkkainen et al., 2020; Whitwell et al., 2012; Zhang et al., 2016). FDG-PET has been implemented as an additional measure correlated with regional atrophy to enhance subtype specificity (Huang et al., 2017; Levin et al., 2021). Such studies link individuals with hippocampal sparing AD, greater global hypometabolism, and reduced executive functioning (Risacher et al., 2017). Importantly, while these classifications are beneficial for appreciating the heterogeneity of AD, there are methodological inconsistencies, and unbiased, multi-modal approaches are needed to better explore disease mechanism (Mohanty et al., 2020).
With the capacity to combine imaging data with genetic information, unique subtypes rooted in clinical phenotypes, omics, and known regions vulnerable to dysregulation can be classified. Understanding subtypes of AD can allow researchers to develop targeted treatments and clinicians to better predict disease course in patients. By clustering differentially expressed genes and conducting weighted network analyses, tau-mediated neurodegeneration, amyloid-β neuroinflammation, and synaptic signaling subtypes were developed (Neff et al., 2021). These subtypes are well represented in complementary mouse models, including the AD-BXD mouse panel (Philip et al., 2021, unpublished personal communication). Since these subtypes were identified as independent of age and disease severity, there is potential for supplemental data types to be incorporated to promote the identification of predictive factors that can then be tested longitudinally. More specially, studying in vivo functional neuroimaging outcomes in combination with other systems biology approaches in transgenic mice may yield important insights regarding the mechanisms that underlie the development of different AD subtypes (Fig. 2).
Fig. 2.
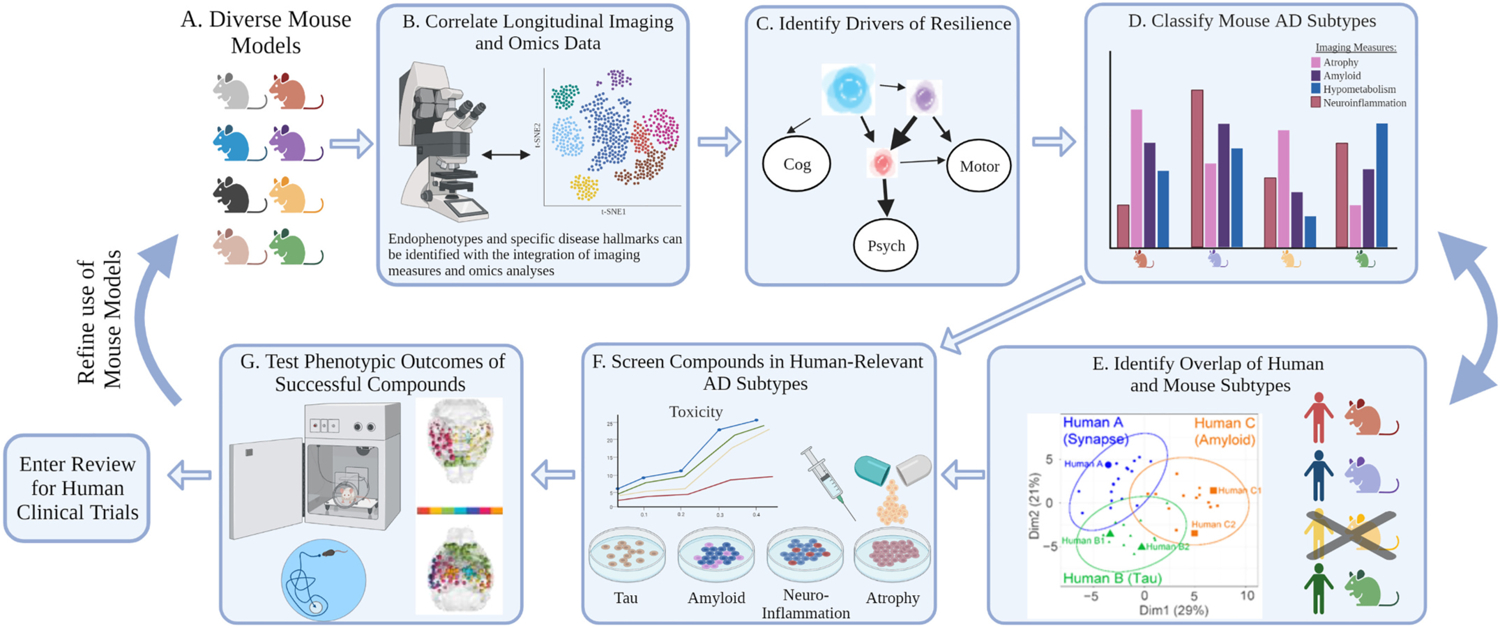
Utility of animal models and systems biology approaches for precision medicine solutions (A) Using a translationally relevant and diverse model mouse population, (B) a vast amount of longitudinal imaging and omics data can and has been collected to develop predictive networks and (C) identify drivers of resilience. (D) These identified modulators can guide the classification of AD subtypes. Subtypes reflect a pattern or prevalence of the collected imaging and omics endophenotypes measures. Single or groups of mouse strains can then be classified into these subtypes based on the display of similar traits, and if available, compared to established human subtypes. Mice sorted into these subtypes can then directly enter the precision medicine discovery drug cycle. (E) We recently tested AD-BXD strains against established human AD subtypes to define human relevant subtypes from hippocampal RNAseq data. (F) Mouse subtypes that appropriately align with human subtypes will then proceed through the pipeline. In vitro models that recapitulate the cellular and molecular profiles of each subtype can be create and implemented to conduct compound screens. Measures of neurodegeneration, synapse number and type, axonal degeneration, and neuron excitability can be quantified to assess the result of each compound on the model system. (G) The efficacy of a select compound’s ability to alter disease course in mice of certain subtypes can then be assessed in vivo with cognitive phenotyping. Overall outcomes of this pipeline will enable precision medicine solutions to be identified per disease subtype and then potentially applied in clinical trials for humans or to refine the selection of mouse strains in future discovery trials. (Created with BioRender.com).
Precision medicine is driven by the application of high-throughput systems biology, powerful computational and statistical modeling tools, and the integration of asymptomatic, preclinical, and clinical datasets to identify and connect novel causal mechanisms of AD (Castrillo et al., 2018; Hampel et al., 2016; Hampel et al., 2018). The resulting subtypes and networks identified foster precise early preclinical detection, effective prevention, and personalized disease modifying treatments (Collins and Varmus, 2015; Hampel et al., 2019b; Uddin et al., 2019). To attain this level of understanding of AD mechanism and manifestation, large-scale model organism experiments that survey translatable AD biomarkers need to be performed (Fig. 2).
5. Future directions of systems biology and use of imaging modalities to evaluate AD progression
Systems biology has the potential to change how AD is defined and translated from bench to bedside. Human studies have demonstrated that identifying structural and functional relationships using in vivo imaging data combined with clinical outcomes has increased our understanding of disease outcomes. Expanding and building upon these studies using model mouse populations will allow researchers better control and the ability to manipulate mechanistic networks across various scales of biology and environmental exposures. Applying systems biology approaches to large reproducible cohorts will be a crucial step toward identifying predictive AD biomarkers, establishing predictive models, and creating precision medicine solutions.
While the benefits of these approaches are clear, there are current challenges to applying imaging modalities and systems biology. Taking a systems biology approach to investigating precision medicine solutions to AD necessitates large sample sizes. Current human studies require hundreds to thousands of individuals to map genetic risk loci or correlate biomarkers with clinical outcomes (Ard and Edland, 2011; Brookmeyer and Abdalla, 2019; Ederer et al., 1993; Grill et al., 2013). To achieve more confident results from genetic mapping, the same is also needed in mouse studies. Large sample sizes are essential to pursue multimodal analyses of varying biological systems and scales. Monetary and time investments can also greatly influence the feasibility to complete these studies, but a well powered study in an appropriate model has the potential to significantly contribute to the understanding and mechanisms of AD. Moreover, applying imaging modalities to large systems biology datasets requires a significant amount of data processing, which presents the opportunity to introduce user bias. To combat this, the field is pushing towards standardization of imaging acquisition and processing methods (Mueller et al., 2005; Whitesell et al., 2019). Even when using identical mouse models and similar imaging approaches, independent labs can achieve varying results for measures like rate of amyloid plaque accumulation or regional levels of atrophy (Kolinger et al., 2021; Mannheim et al., 2018; Morbelli and Bauckneht, 2018; Osborne et al., 2017). This lack of consistency makes it difficult to combine and compare results, and for these reasons, all processing parameters should be disclosed to promote reproducibility (Eisenstein, 2020). The creation of centralized data portals and collaborative efforts have promoted this type of synchronicity (Hodes and Buckholtz, 2016; Kitano, 2002a). These sites provide researchers a platform to collaborate, contribute their own data, or to analyze data from other groups (Table 3). Furthermore, another area the AD imaging field can improve upon is in the analysis of subjective regions of interest (Simpson et al., 2021). Manual stereology or delineation is based on the user’s experience and anatomical knowledge. Even the scope and use of machine learning and artificial intelligence guided methods is biased by users as they set training parameters. Use of more automated pipelines and standard brain atlases aid in reducing the subjectivism of quantifying and reporting region specific measures (Bjerke et al., 2018; Boline et al., 2008; Hawrylycz et al., 2011; Hjornevik et al., 2007; Johnson et al., 2010; Wang et al., 2020). High throughput studies including many regions of interest or brain-wide approaches can give a more comprehensive large-scale look at the changes that occur with disease status.
Table 3.
General multi-omics and AD specific data portals and platforms.
| Data portal | Portal description | Citation |
|---|---|---|
| OpenNeuro |
|
https://openneuro.org/ |
| Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) |
|
http://enigma.ini.usc.edu/ |
| Encyclopedia of DNA Elements (ENCODE) |
|
https://www.encodeproject.org/ |
| Synapse by Sage Bionetworks |
|
https://www.synapse.org/ |
| Accelerating Medicines Partnership Program for Alzheimer’s Disease (AMP-AD) Knowledge Portal |
|
https://adknowledgeportal.synapse.org/ |
| AGORA |
|
https://sagebionetworks.org/tools_resources/agora/ |
| Alzheimer’s Disease and Healthy Aging Data Portal |
|
https://www.cdc.gov/aging/agingdata/ |
| Single-cell RNA-Seq database for Alzheimer’s disease (scREAD) |
|
https://bmbls.bmi.osumc.edu/scread/ |
| Alzheimer’s Disease Neuroimaging Initiative (ADNI) |
|
http://adni.loni.usc.edu/ |
| The National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS) |
|
https://www.niagads.org/ |
| National Alzheimer’s Coordinating Center (NACC) |
|
https://www.alz.washington.edu/ |
| Global Alzheimer’s Association Interactive Network (GAAIN) |
|
http://www.gaain.org/ |
| The Rush Alzheimer’s Disease Center (RADC) Research Resource Sharing Hub |
|
https://www.radc.rush.edu/ |
| Alzheimer’s Disease Cooperative Study (ADRCS) |
|
https://www.adcs.org/ |
6. Conclusion
Integrating multiple imaging modalities with the various omics of systems biology permits a thorough investigation of AD and its interacting components across various biological systems and scales. The need to incorporate many imaging and omics methods to study individual humans or mouse strains pays tribute to the complexity of this disease. Modelling AD in mice offers the advantage of parsing out the interacting components underlying AD manifestation in a controlled fashion. Evaluating a wide variety of strains, samples, and methods within translationally relevant mouse panels enables researchers to untangle the mechanisms perturbed in the different subtypes of AD. AD progression is viewed as a spectrum, but patterns of interacting levels of systems biology (e.g. genomic, transcriptomic, or metabolomics relationship to neural connectivity) are evident and are the foundation for classifying mouse models and human individuals into AD subtypes. Categorizing individuals’ disease subtypes paves the way towards fully understanding the complexity of AD, how it manifests differently among individuals, and eventually, precision medicine solutions.
Acknowledgments
This study is part of the National Institute on Aging Resilience-AD program and is supported through the NIA grant award R01AG057914 to Catherine Kaczorowski. The authors would also like to thank Dr. Kristen O’Connell, Dr. Niran Hadad, Dr. Amy Dunn, Dr. Maria Telpoukhovskaia, and Dr. Jillian King for their thoughtful review and editing.
Footnotes
Declaration of Competing Interest
None.
References
- 2019 Alzheimer’s Disease Facts and Figures, 2019. Alzheimers Dement. 15 (3), 321–387. 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 2020 Alzheimer’s Disease Facts and Figures, 2020. Alzheimers Dement. 16 (3), 391–460. 10.1002/alz.12068. [DOI] [Google Scholar]
- Adil A, Kumar V, Jan AT, Asger M, 2021. Single-cell transcriptomics: current methods and challenges in data acquisition and analysis. Front. Neurosci 15, 591122 10.3389/fnins.2021.591122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang S-C, Barrio JR, 2001. Binding characteristics of radiofluorinated 6-Dialkylamino-2-Naphthylethylidene derivatives as positron emission tomography imaging probes for β-Amyloid plaques in Alzheimer’s disease. J. Neurosci 21 (24), RC189. 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn AC, Tewari M, Poon C-S, Phillips RS, 2006. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med. 3 (6), e208 10.1371/journal.pmed.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello Bowles EJ, Crane PK, Walker RL, Chubak J, LaCroix AZ, Anderson ML, Rosenberg D, Keene CD, Larson EB, 2019. Cognitive resilience to Alzheimer’s disease pathology in the human brain. J. Alzheimers Dis 68 (3), 1071–1083. 10.3233/JAD-180942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Cummings J, Jack CR, Morris JC, Sperling R, Frölich L, Jones RW, Dowsett SA, Matthews BR, Raskin J, Scheltens P, Dubois B, 2017. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther 9 (1), 60. 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberghina L, Colangelo AM, 2006. The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci. 7 (Suppl. 1), S2. 10.1186/1471-2202-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4 (3), 316–329. 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Cook JJ, Delpratt N, Nouls JC, Gu B, McNamara JO, Avants BB, Johnson GA, Badea A, 2019. Small animal multivariate brain analysis (SAMBA) – a high throughput pipeline with a validation framework. Neuroinformatics 17 (3), 451–472. 10.1007/s12021-018-9410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SJ, Fulton-Howard B, Goate A, 2020. Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol. 19 (4), 326–335. 10.1016/S1474-4422(19)30435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ard MC, Edland SD, 2011. Power calculations for clinical trials in Alzheimer’s disease. J. Alzheimers Dis 26 (Suppl. 3), 369–377. 10.3233/JAD-2011-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Convit A, Bachman AH, 2016. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J. Alzheimer’s Disease JAD 50 (3), 847–857. 10.3233/JAD-150780. [DOI] [PubMed] [Google Scholar]
- Argelaguet R, Velten B, Arnol D, Dietrich S, Zenz T, Marioni JC, Buettner F, Huber W, Stegle O, 2018. Multi-omics factor analysis-a framework for unsupervised integration of multi-omics data sets. Mol. Syst. Biol 14 (6), e8124 10.15252/msb.20178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA, Wood L, 1994. The identification of pathological subtypes of Alzheimer’s disease using cluster analysis. Acta Neuropathol. 88 (1), 60–66. 10.1007/BF00294360. [DOI] [PubMed] [Google Scholar]
- Ashbrook DG, Williams RW, Lu L, Stein JL, Hibar DP, Nichols TE, Medland SE, Thompson PM, Hager R, 2014. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. BMC Genomics 15 (1), 850. 10.1186/1471-2164-15-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbrook DG, Arends D, Prins P, Mulligan MK, Roy S, Williams EG, Lutz CM, Valenzuela A, Bohl CJ, Ingels JF, McCarty MS, Centeno AG, Hager R, Auwerx J, Lu L, Williams RW, 2021. A platform for experimental precision medicine: the extended BXD mouse family. Cell Syst. 10.1016/j.cels.2020.12.002.S2405471220305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, Benner C, Liu D, Locke AE, Balasubramanian S, Yadav A, Banerjee N, Gillies C, Damask A, Liu S, Bai X, Hawes A, Maxwell E, Gurski L, Watanabe K, Kosmicki JA, Rajagopal V, Mighty J, Regeneron Genetics, C., Discovehr, Jones M, Mitnaul L, Stahl E, Coppola G, Jorgenson E, Habegger L, Salerno WJ, Shuldiner AR, Lotta LA, Overton JD, Cantor MN, Reid JG, Yancopoulos G, Kang HM, Marchini J, Baras A, Abecasis GR, Ferreira MA, 2021. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 10.1038/s41586-021-04103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea A, Johnson GA, Jankowsky JL, 2010. Remote sites of structural atrophy predict later amyloid formation in a mouse model of Alzheimer’s disease. NeuroImage 50 (2), 416–427. 10.1016/j.neuroimage.2009.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea A, Delpratt NA, Anderson RJ, Dibb R, Qi Y, Wei H, Liu C, Wetsel WC, Avants BB, Colton C, 2019. Multivariate MR biomarkers better predict cognitive dysfunction in mouse models of Alzheimer’s disease. Magn. Reson. Imaging 60, 52–67. 10.1016/j.mri.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhwar A, Lerch JP, Hamel E, Sled JG, 2013. Impaired structural correlates of memory in Alzheimer’s disease mice. Neuroimage Clin. 3, 290–300. 10.1016/j.nicl.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10 (9), 819–828. 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 62 (6), 685–691. 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC, 1996. Weight loss precedes dementia in community-dwelling older adults. J. Am. Geriatr. Soc 44 (10), 1147–1152. 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Basaia S, Agosta F, Wagner L, Canu E, Magnani G, Santangelo R, Filippi M, Neuroimaging, Alzheimer’s Disease, I., 2019. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. Neuroimage Clin. 21, 101645 10.1016/j.nicl.2018.101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheera S, Sai Ram MS, 2019. Convolution neural network-based Alzheimer’s disease classification using hybrid enhanced independent component analysis based segmented gray matter of T2 weighted magnetic resonance imaging with clinical valuation. Alzheimers Dement (N Y) 5, 974–986. 10.1016/j.trci.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Bahri MA, Meyer F, Manard M, Delhaye E, Plenevaux A, Becker G, Seret A, Mella C, Giacomelli F, Degueldre C, Balteau E, Luxen A, Salmon E, 2020. In vivo imaging of synaptic loss in Alzheimer’s disease with [18F]UCB-H positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 47 (2), 390–402. 10.1007/s00259-019-04461-x. [DOI] [PubMed] [Google Scholar]
- Bellenguez C, Küçükali F, Jansen I, Andrade V, Moreno-Grau S, Amin N, Naj AC, Grenier-Boley B, Campos-Martin R, Holmans PA, Boland A, Kleineidam L, Damotte V, Van Der Lee SJ, Kuulasmaa T, Yang Q, De Rojas I, Bis JC, Yaqub A, Prokic I, Costa MR, Chapuis J, Ahmad S, Giedraitis V, Boada M, Aarsland D, García-González P, Abdelnour C, Alarcón-Martín E, Alegret M, Alvarez I, Álvarez V, Armstrong NJ, Tsolaki A, Antúnez C, Appollonio I, Arcaro M, Archetti S, Pastor AA, Arosio B, Athanasiu L, Bailly H, Banaj N, Baquero M, Beĺen Pastor A, Benussi L, Berr C, Besse C, Bessi V, Binetti G, Bizzarro A, Alcolea D, Blesa R, Borroni B, Boschi S, Bossù P, Bråthen G, Bresner C, Brookes KJ, Brusco LI, Bûrger K, Bullido MJ, Burholt V, Bush WS, Calero M, Dufouil C, Carracedo Á, Cecchetti R, Cervera-Carles L, Charbonnier C, Chillotti C, Brodaty H, Ciccone S, Claassen JAHR, Clark C, Conti E, Corma-Gómez A, Costantini E, Custodero C, Daian D, Dalmasso MC, Daniele A, Dardiotis E, Dartigues J-F, De Deyn PP, De Paiva Lopes K, De Witte LD, Debette S, Deckert J, Del Ser T, Denning N, Destefano A, Dichgans M, Diehl-Schmid J, Diez-Fairen M, Rossi PD, Djurovic S, Duron E, Düzel E, Engelborghs S, Escott-Price V, Espinosa A, Buiza-Rueda D, Ewers M, Tagliavini F, Nielsen SF, Farotti L, Fenoglio C, Fernández-Fuertes M, Hardy J, Ferrari R, Ferreira CB, Ferri E, Fin B, Fischer P, Fladby T, Fließbach K, Fortea J, Fostinelli S, Fox NC, Franco-Macías E, Frank-García A, Froelich L, Galimberti D, García-Alberca JM, Garcia-Madrona S, García-Ribas G, Chene G, Ghidoni R, Giegling I, Giaccone G, Goldhardt O, Gonźalez-Pérez A, Graff C, Grande G, Green E, Grimmer T, Grünblatt E, Guetta-Baranes T, Haapasalo A, Hadjigeorgiou G, Haines JL, Hamilton-Nelson KL, Hampel H, Hanon O, Hartmann AM, Hausner L, Harwood J, Heilmann-Heimbach S, Helisalmi S, Heneka MT, Hernández I, Herrmann MJ, Hoffmann P, Holmes C, Holstege H, Vilas RH, Hulsman M, Humphrey J, Biessels GJ, Johansson C, Kehoe PG, Kilander L, Ståhlbom AK, Kivipelto M, Koivisto A, Kornhuber J, Kosmidis MH, Kuksa PP, Kunkle BW, Lage C, Laukka EJ, Lauria A, Lee C-Y, Lehtisalo J, Satizabal CL, Lerch O, Lleó A, Lopez R, Lopez O, De Munain AL, Love S, Löwemark M, Luckcuck L, Macías J, Macleod CA, Maier W, Mangialasche F, Spallazzi M, Marquíe M, Marshall R, Martin ER, Martín Montes A, Rodríguez CM, Masullo C, Mayeux R, Mead S, Mecocci P, Medina M, Meggy A, Mendoza S, Meńendez-González M, Mir P, Periñán MT, Mol M, Molina-Porcel L, Montrreal L, Morelli L, Moreno F, Morgan K, Nöthen MM, Muchnik C, Nacmias B, Ngandu T, Nicolas G, Nordestgaard BG, Olaso R, Orellana A, Orsini M, Ortega G, Padovani A, Caffarra P, Papenberg G, Parnetti L, Pasquier F, Pastor P, Pérez-Cordón A, Pérez-Tur J, Pericard P, Peters O, Pijnenburg YAL, Pineda JA, Piñol-Ripoll G, Pisanu C, Polak T, Popp J, Posthuma D, Priller J, Puerta R, Quenez O, Quintela I, Thomassen JQ, Ŕabano A, Rainero I, Ramakers I, Real LM, Reinders MJT, Riedel-Heller S, Riederer P, Rodriguez-Rodriguez E, Rongve A, Allende IR, Rosende-Roca M, Royo JL, Rubino E, Rujescu D, Sáez ME, Sakka P, Saltvedt I, Sanabria Á, Sánchez-Arjona MB, Sanchez-Garcia F, Mehrabian S, Sánchez-Juan P, Sánchez-Valle R, Sando SB, Scamosci M, Scarmeas N, Scarpini E, Scheltens P, Scherbaum N, Scherer M, Schmid M, Schneider A, Schott JM, Selbæk G, Sha J, Shadrin AA, Skrobot O, Snijders GJL, Soininen H, Solfrizzi V, Solomon A, Sorbi S, Sotolongo-Grau O, Spalletta G, Spottke A, Squassina A, Tartari JP, Tárraga L, Tesí N, Thalamuthu A, Tegos T, Traykov L, Tremolizzo L, Tybjærg-Hansen A, Uitterlinden A, Ullgren A, Ulstein I, Valero S, Van Broeckhoven C, Van Der Lugt A, Van Dongen J, Van Rooij J, Van Swieten J, Vandenberghe R, Verhey F, Vidal J-S, Vogelgsang J, Vyhnalek M, Wagner M, Wallon D, Wang L-S, Wang R, Weinhold L, Wiltfang J, Windle G, Woods B, Yannakoulia M, Zhao Y, Zulaica M, Serrano-Rios M, Seripa D, Stordal E, Farrer LA, Psaty BM, Ghanbari M, Raj T, Sachdev P, Mather K, Jessen F, Ikram MA, De Mendonça A, Hort J, Tsolaki M, Pericak-Vance MA, Amouyel P, Williams J, Frikke-Schmidt R, Clarimon J, Deleuze J-F, Rossi G, Seshadri S, Andreassen OA, Ingelsson M, Hiltunen M, Sleegers K, Schellenberg GD, Van Duijn CM, Sims R, Van Der Flier WM, Ruiz A, Ramirez A, Lambert J-C, 2020. New Insights on the Genetic Etiology of Alzheimer’s and Related Dementia. Cold Spring Harbor Laboratory. [Google Scholar]
- Belloy ME, Napolioni V, Greicius MD, 2019. A quarter century of APOE and Alzheimer’s disease: progress to date and the path forward. Neuron 101 (5), 820–838. 10.1016/j.neuron.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS, 2006. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66 (12), 1837. 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA, 2018. Religious orders study and rush memory and aging project. J. Alzheimer’s Disease JAD 64 (Suppl. 1), S161–S189. 10.3233/JAD-179939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P, 2006. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 (1), 64–74. 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bjerke IE, Øvsthus M, Andersson KA, Blixhavn CH, Kleven H, Yates SC, Puchades MA, Bjaalie JG, Leergaard TB, 2018. Navigating the murine brain: toward best practices for determining and documenting neuroanatomical locations in experimental studies. Front. Neuroanat 12, 82. 10.3389/fnana.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekel J, Chilton JM, Cooke IR, Horvatovich PL, Jagtap PD, Kall L, Lehtio J, Lukasse P, Moerland PD, Griffin TJ, 2015. Multi-omic data analysis using galaxy. Nat. Biotechnol 33 (2), 137–139. 10.1038/nbt.3134. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Wu C, Barbas H, Bokil H, Bota M, Breiter HC, Cline HT, Doyle JC, Freed PJ, Greenspan RJ, Haber SN, Hawrylycz M, Herrera DG, Hilgetag CC, Huang ZJ, Jones A, Jones EG, Karten HJ, Kleinfeld D, Kotter R, Lester HA, Lin JM, Mensh BD, Mikula S, Panksepp J, Price JL, Safdieh J, Saper CB, Schiff ND, Schmahmann JD, Stillman BW, Svoboda K, Swanson LW, Toga AW, Van Essen DC, Watson JD, Mitra PP, 2009. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Comput. Biol 5 (3), e1000334 10.1371/journal.pcbi.1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boline J, Lee EF, Toga AW, 2008. Digital atlases as a framework for data sharing. Front. Neurosci 2 (1), 100–106. 10.3389/neuro.01.012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW, 2000. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med 343 (7), 450–456. 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter C, Henniges P, Franke TN, Irwin C, Sahlmann CO, Sichler ME, Beindorff N, Bayer TA, Bouter Y, 2018. (18)F-FDG-PET detects drastic changes in brain metabolism in the Tg4-42 model of Alzheimer’s disease. Front. Aging Neurosci 10, 425. 10.3389/fnagi.2018.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA, 2013. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann. Neurol 74 (3), 478–489. 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82 (4), 239–259. 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112 (4), 389–404. 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brademan DR, Miller IJ, Kwiecien NW, Pagliarini DJ, Westphall MS, Coon JJ, Shishkova E, 2020. Argonaut: A web platform for collaborative multi-omic data visualization and exploration. Patterns (N Y) 1 (7). 10.1016/j.patter.2020.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, Mccarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM, 2016. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med 8 (338) 10.1126/scitranslmed.aaf2362, 338ra366–338ra366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, 2019. Design and sample size considerations for Alzheimer’s disease prevention trials using multistate models. Clin. Trials 16 (2), 111–119. 10.1177/1740774518816323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RKJ, Bohnen NI, Wong KK, Minoshima S, Frey KA, 2014. Brain PET in suspected dementia: patterns of altered FDG metabolism. RadioGraphics 34 (3), 684–701. 10.1148/rg.343135065. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA, 2005. Change in body mass index and risk of incident Alzheimer disease. Neurology 65 (6), 892–897. 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- Budin F, Hoogstoel M, Reynolds P, Grauer M, O’Leary-Moore SK, Oguz I, 2013. Fully automated rodent brain MR image processing pipeline on a Midas server: from acquired images to region-based statistics. Front. Neuroinform 7, 15. 10.3389/fninf.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold K-H, Haass C, Staufenbiel M, Konnerth A, Garaschuk O, 2008. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321 (5896), 1686–1689. 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A, 2012. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci 109 (22), 8740–8745. 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MS, Kim SE, Park J, Yi D, Choe YM, Sohn BK, Choi HJ, Baek H, Han JY, Woo JI, Lee DY, Alzheimer’s Disease Neuroimaging, I, 2015. Heterogeneity of regional brain atrophy patterns associated with distinct progression rates in Alzheimer’s disease. PLoS One 10 (11), e0142756. 10.1371/journal.pone.0142756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Li S, Matuskey D, Nabulsi N, Huang Y, 2019. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases. Neurosci. Lett 691, 44–50. 10.1016/j.neulet.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Hou J, Ping J, Cai D, 2018. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener 13 (1), 64. 10.1186/s13024-018-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW, 2011. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol. Aging 32 (4), 572–580. 10.1016/j.neurobiolaging.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo JI, Lista S, Hampel H, Ritchie CW, 2018. Systems biology methods for Alzheimer’s disease research toward molecular signatures, subtypes, and stages and precision medicine: application in cohort studies and trials. Methods Mol. Biol 1750, 31–66. 10.1007/978-1-4939-7704-8_3. [DOI] [PubMed] [Google Scholar]
- Chaney A, Bauer M, Bochicchio D, Smigova A, Kassiou M, Davies KE, Williams SR, Boutin H, 2018. Longitudinal investigation of neuroinflammation and metabolite profiles in the APPswe xPS1Deltae9 transgenic mouse model of Alzheimer’s disease. J. Neurochem 144 (3), 318–335. 10.1111/jnc.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Shcherbinin S, Southekal S, Devous MD, Mintun M, Murray ME, Miller BB, Schwarz AJ, 2019. Tau subtypes of Alzheimer’s disease determined in vivo using flortaucipir PET imaging. J. Alzheimers Dis 71 (3), 1037–1048. 10.3233/JAD-190264. [DOI] [PubMed] [Google Scholar]
- Chen C, Liang Z, Zhou B, Li X, Lui C, Ip NY, Qu JY, 2018a. In vivo near-infrared two-photon imaging of amyloid plaques in deep brain of Alzheimer’s disease mouse model. ACS Chem. Neurosci 9 (12), 3128–3136. 10.1021/acschemneuro.8b00306. [DOI] [PubMed] [Google Scholar]
- Chen G-F, Xu T-H, Yan Y, Zhou Y-R, Jiang Y, Melcher K, Xu HE, 2017. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin 38 (9), 1205–1235. 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, Najafzadeh S, Ropchan J, Lu Y, McDonald JW, Michalak HR, Nabulsi NB, Arnsten AFT, Huang Y, Carson RE, van Dyck CH, 2018b. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. 75 (10), 1215–1224. 10.1001/jamaneurol.2018.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-T, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Lálaková J, Kühnemund M, Voytyuk I, Wolfs L, Mancuso R, Salta E, Balusu S, Snellinx A, Munck S, Jurek A, Fernandez Navarro J, Saido TC, Huitinga I, Lundeberg J, Fiers M, De Strooper B, 2020. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182 (4), 976–991.e919. 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, 2012. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life study. Am. J. Geriatr. Psychiatry 20 (7), 635–639. 10.1097/JGP.0b013e31823032a9. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, Lyoo CH, Ryu YH, Lee MS, 2016. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 87 (4), 375–383. 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- Chouliaras L, Sierksma AS, Kenis G, Prickaerts J, Lemmens MA, Brasnjevic I, van Donkelaar EL, Martinez-Martinez P, Losen M, De Baets MH, Kholod N, van Leeuwen F, Hof PR, van Os J, Steinbusch HW, van den Hove DL, Rutten BP, 2010. Gene-environment interaction research and transgenic mouse models of Alzheimer’s disease. Int. J. Alzheimers Dis 2010 10.4061/2010/859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Varmus H, 2015. A new initiative on precision medicine. N. Engl. J. Med 372 (9), 793–795. 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Perez LM, Ibanez KR, Suarez M, Torroella K, Acuna K, Ofori E, Levites Y, Vaillancourt DE, Golde TE, Chakrabarty P, Febo M, 2019. Neurite orientation dispersion and density imaging reveals white matter and hippocampal microstructure changes produced by Interleukin-6 in the TgCRND8 mouse model of amyloidosis. NeuroImage 202, 116138. 10.1016/j.neuroimage.2019.116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 (5123), 921–923. 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, Benitez BA, Jeng AT, Skorupa T, Carrell D, Bertelsen S, Bailey M, McKean D, Shulman JM, De Jager PL, Chibnik L, Bennett DA, Arnold SE, Harold D, Sims R, Gerrish A, Williams J, Van Deerlin VM, Lee VM, Shaw LM, Trojanowski JQ, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Peskind ER, Galasko D, Fagan AM, Holtzman DM, Morris JC, Consortium, G, Alzheimer’s Disease Neuroimaging I, Alzheimer Disease Genetic, C, Goate AM, 2013. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron 78 (2), 256–268. 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseĺenyi Z, Jönhagen ME, Forsberg A, Halldin C, Julin P, Schou M, Johnström P, Varnäs K, Svensson S, Farde L, 2012. Clinical validation of 18FAZD4694, an amyloid-β-specific PET radioligand. J. Nucl. Med 53 (3), 415–424. 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- Cui MY, Lin Y, Sheng JY, Zhang X, Cui RJ, 2018. Exercise intervention associated with cognitive improvement in Alzheimer’s disease. Neural Plasticity 2018, 9234105. 10.1155/2018/9234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, 2019. The role of biomarkers in Alzheimer’s disease drug development. Adv. Exp. Med. Biol 1118, 29–61. 10.1007/978-3-030-05542-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K, 2014. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther 6 (4), 37. 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Ma Y, McCabe C, Xu J, Vardarajan BN, Felsky D, Klein H-U, White CC, Peters MA, Lodgson B, Nejad P, Tang A, Mangravite LM, Yu L, Gaiteri C, Mostafavi S, Schneider JA, Bennett DA, 2018. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci. Data 5 (1), 180142. 10.1038/sdata.2018.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas AG, Viña J, Gomez-Cabrera MC, 2020. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci 9 (5), 394–404. 10.1016/j.jshs.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J, 2001. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol. Aging 22 (4), 529–539. 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Deming Y, Dumitrescu L, Barnes LL, Thambisetty M, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Huentelman M, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Moghekar A, Del Aguila JL, Fernandez MV, Budde J, Hassenstab J, Fagan AM, Riemenschneider M, Petersen RC, Minthon L, Chao MJ, Van Deerlin VM, Lee VM-Y, Shaw LM, Trojanowski JQ, Peskind ER, Li G, Davis LK, Sealock JM, Cox NJ, ADNI ADGC, Goate AM, Bennett DA, Schneider JA, Jefferson AL, Cruchaga C, Hohman TJ, 2018. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol. 136 (6), 857–872. 10.1007/s00401-018-1881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Briggman KL, Helmstaedter M, 2012. Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat. Rev. Neurosci 13 (5), 351–358. 10.1038/nrn3169. [DOI] [PubMed] [Google Scholar]
- Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC, 2020. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology 95 (4), e374–e383. 10.1212/WNL.0000000000009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA, 2008. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46 (6), 1624–1635. 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis SI, Liparas D, Neuroimaging, Alzheimer’s Disease, I., 2018. How random is the random forest? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database. Neural Regen. Res 13 (6), 962–970. 10.4103/1673-5374.233433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Troncoso J, 2011. Asymptomatic Alzheimer’s disease: a prodrome or a state of resilience? Curr. Alzheimer Res 8 (4), 330–335. 10.2174/156720511795745348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E, Wisniewski T, 2017. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 133 (2), 155–175. 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues J-F, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert M-O, Holtzman DM, Kivipelto M, Lista S, Molinuevo J-L, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR, 2016. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimer’s Dement. J. Alzheimer’s Assoc 12 (3), 292–323. 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Bush WS, Gifford KA, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Deming Y, Chao MJ, Huentelman M, Martin ER, Hamilton-Nelson K, Shaw LM, Trojanowski JQ, Peskind ER, Cruchaga C, Pericak-Vance MA, Goate AM, Cox NJ, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Initiative, F. T. A. S. D. G. C. A. T. A. S. D. N, Bennett DA, Schneider JA, Jefferson AL, Hohman TJ, 2019. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain 142 (9), 2581–2589. 10.1093/brain/awz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, Mahoney ER, Mukherjee S, Lee ML, Bush WS, Engelman CD, Lu Q, Fardo DW, Trittschuh EH, Mez J, Kaczorowski C, Hernandez Saucedo H, Widaman KF, Buckley R, Properzi M, Mormino E, Yang HS, Harrison T, Hedden T, Nho K, Andrews SJ, Tommet D, Hadad N, Sanders RE, Ruderfer DM, Gifford KA, Moore AM, Cambronero F, Zhong X, Raghavan NS, Vardarajan B, Alzheimer’s Disease Neuroimaging, I, Alzheimer’s Disease Genetics Consortium, A. S. T, Pericak-Vance MA, Farrer LA, Wang LS, Cruchaga C, Schellenberg G, Cox NJ, Haines JL, Keene CD, Saykin AJ, Larson EB, Sperling RA, Mayeux R, Bennett DA, Schneider JA, Crane PK, Jefferson AL, Hohman TJ, 2020. Genetic variants and functional pathways associated with resilience to Alzheimer’s disease. Brain 143 (8), 2561–2575. 10.1093/brain/awaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AR, O’Connell KMS, Kaczorowski CC, 2019. Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci. Biobehav. Rev 103, 73–80. 10.1016/j.neubiorev.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Wiesner T, Gardner MA, Robitaille LE, Bilodeau A, Gagne C, De Koninck P, Lavoie-Cardinal F, 2018. A machine learning approach for online automated optimization of super-resolution optical microscopy. Nat. Commun 9 (1), 5247. 10.1038/s41467-018-07668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederer F, Church TR, Mandel JS, 1993. Sample sizes for prevention trials have been too small. Am. J. Epidemiol 137 (7), 787–796. 10.1093/oxfordjournals.aje.a116739. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ, 2008. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis 32 (3), 412–419. 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M, Elf J, Aurell E, Sandberg R, Tegner J, 2003. Systems biology is taking off. Genome Res. 13 (11), 2377–2380. 10.1101/gr.1763203. [DOI] [PubMed] [Google Scholar]
- Eid A, Mhatre I, Richardson JR, 2019. Gene-environment interactions in Alzheimer’s disease: a potential path to precision medicine. Pharmacol. Ther 199, 173–187. 10.1016/j.pharmthera.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein M, 2020. Smart solutions for automated imaging. Nat. Methods 17 (11), 1075–1079. 10.1038/s41592-020-00988-2. [DOI] [PubMed] [Google Scholar]
- Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, Marchini J, Smith SM, 2018. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562 (7726), 210–216. 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh SSM, Chimusa ER, Mulder NJ, Crimi A, 2020. Genome-wide association study of brain connectivity changes for Alzheimer’s disease. Sci. Rep 10 (1), 1433. 10.1038/s41598-020-58291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ, 2019. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16 (1), 111–116. 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola MF, Dyakin VV, Lee SP, Bogart A, Babb JS, Duff K, Nixon R, Helpern JA, 2007. Quantitative MRI reveals aging-associated T2 changes in mouse models of Alzheimer’s disease. NMR Biomed. 20 (3), 343–351. 10.1002/nbm.1163. [DOI] [PubMed] [Google Scholar]
- Febo M, Perez PD, Ceballos-Diaz C, Colon-Perez LM, Zeng H, Ofori E, Golde TE, Vaillancourt DE, Chakrabarty P, 2020. Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-gamma. Brain Struct. Funct 225 (1), 427–439. 10.1007/s00429-019-02017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Lau C, Ng L, Li Y, Kuan L, Sunkin SM, Dang C, Hawrylycz M, 2015. Exploration and visualization of connectivity in the adult mouse brain. Methods 73, 90–97. 10.1016/j.ymeth.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Ferreira D, Verhagen C, Hernandez-Cabrera JA, Cavallin L, Guo CJ, Ekman U, Muehlboeck JS, Simmons A, Barroso J, Wahlund LO, Westman E, 2017. Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci. Rep 7, 46263. 10.1038/srep46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Kulminski AM, 2019. The Alzheimer’s disease exposome. Alzheimers Dement. 15 (9), 1123–1132. 10.1016/j.jalz.2019.06.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Nabulsi NB, Mercier J, Lin SF, Chen MK, Matuskey D, Gallezot JD, Henry S, Hannestad J, Huang Y, Carson RE, 2018. Kinetic evaluation and test-retest reproducibility of [(11)C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J. Cereb. Blood Flow Metab 38 (11), 2041–2052. 10.1177/0271678X17724947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke C, Blume T, Zott B, Shi Y, Deussing M, Peters F, Schmidt C, Kleinberger G, Lindner S, Gildehaus FJ, Beyer L, von Ungern-Sternberg B, Bartenstein P, Ozmen L, Baumann K, Dorostkar MM, Haass C, Adelsberger H, Herms J, Rominger A, Brendel M, 2019. Early and longitudinal microglial activation but not amyloid accumulation predicts cognitive outcome in PS2APP mice. J. Nucl. Med 60 (4), 548–554. 10.2967/jnumed.118.217703. [DOI] [PubMed] [Google Scholar]
- Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, Di Chiro G, 1983. Alzheimer’s disease: focal cortical changes shown by positron emission tomography. Neurology 33 (8), 961–965. 10.1212/wnl.33.8.961. [DOI] [PubMed] [Google Scholar]
- Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, DeCarli CS, Turner RS, Koeppe RA, Higdon R, Minoshima S, 2007. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain: A J. Neurol 130 (Pt 10), 2616–2635. 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- Franco R, Cedazo-Minguez A, 2014. Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Front. Pharmacol 5 10.3389/fphar.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Vladimirov N, Kawashima T, Mu Y, Sofroniew NJ, Bennett DV, Rosen J, Yang CT, Looger LL, Ahrens MB, 2014. Mapping brain activity at scale with cluster computing. Nat. Methods 11 (9), 941–950. 10.1038/nmeth.3041. [DOI] [PubMed] [Google Scholar]
- Furney SJ, Simmons A, Breen G, Pedroso I, Lunnon K, Proitsi P, Hodges A, Powell J, Wahlund LO, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Spenger C, Lathrop M, Shen L, Kim S, Saykin AJ, Weiner MW, Lovestone S, Alzheimer’s Disease Neuroimaging. I, AddNeuroMed, C, 2011. Genome-wide association with MRI atrophy measures as a quantitative trait locus for Alzheimer’s disease. Mol. Psychiatry 16 (11), 1130–1138. 10.1038/mp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth D, Vaissiere T, Tzortzi O, Xuan Y, Martin A, Lazaridis I, Spigolon G, Fisone G, Tomer R, Deisseroth K, Carlen M, Miller CA, Rumbaugh G, Meletis K, 2018. An interactive framework for whole-brain maps at cellular resolution. Nat. Neurosci 21 (1), 139–149. 10.1038/s41593-017-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail Canter R, Huang WC, Choi H, Wang J, Ashley Watson L, Yao CG, Abdurrob F, Bousleiman SM, Young JZ, Bennett DA, Delalle I, Chung K, Tsai LH, 2019. 3D mapping reveals network-specific amyloid progression and subcortical susceptibility in mice. Commun. Biol 2, 360. 10.1038/s42003-019-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL, 2006. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63 (2), 168. 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Nourhashemi F, Andrieu S, de Glisezinski I, Ousset PJ, Riviere D, Albarede JL, Vellas B, 2000. Weight loss in Alzheimer disease. Am. J. Clin. Nutr 71 (2), 637S–642S. 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- Götz J, Bodea L-G, Goedert M, 2018. Rodent models for Alzheimer disease. Nat. Rev. Neurosci 19 (10), 583–598. 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- Granic I, Masman MF, Luiten PG, Eisel UL, 2010. Braak staging in mouse models of Alzheimer’s disease. Am. J. Pathol 177 (4), 1603–1605. 10.2353/ajpath.2010.100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Ching CRK, McMahon MAB, Shatokhina N, Zsembik LCP, Thomopoulos SI, Zhu AH, Strike LT, Agartz I, Alhusaini S, Almeida MAA, Alnaes D, Amlien IK, Andersson M, Ard T, Armstrong NJ, Ashley-Koch A, Atkins JR, Bernard M, Brouwer RM, Buimer EEL, Bulow R, Burger C, Cannon DM, Chakravarty M, Chen Q, Cheung JW, Couvy-Duchesne B, Dale AM, Dalvie S, de Araujo TK, de Zubicaray GI, de Zwarte SMC, den Braber A, Doan NT, Dohm K, Ehrlich S, Engelbrecht HR, Erk S, Fan CC, Fedko IO, Foley SF, Ford JM, Fukunaga M, Garrett ME, Ge T, Giddaluru S, Goldman AL, Green MJ, Groenewold NA, Grotegerd D, Gurholt TP, Gutman BA, Hansell NK, Harris MA, Harrison MB, Haswell CC, Hauser M, Herms S, Heslenfeld DJ, Ho NF, Hoehn D, Hoffmann P, Holleran L, Hoogman M, Hottenga JJ, Ikeda M, Janowitz D, Jansen IE, Jia T, Jockwitz C, Kanai R, Karama S, Kasperaviciute D, Kaufmann T, Kelly S, Kikuchi M, Klein M, Knapp M, Knodt AR, Kramer B, Lam M, Lancaster TM, Lee PH, Lett TA, Lewis LB, Lopes-Cendes I, Luciano M, Macciardi F, Marquand AF, Mathias SR, Melzer TR, Milaneschi Y, Mirza Schreiber N, Moreira JCV, Muhleisen TW, Muller-Myhsok B, Najt P, Nakahara S, Nho K, Olde Loohuis LM, Orfanos DP, Pearson JF, Pitcher TL, Putz B, Quide Y, Ragothaman A, Rashid FM, Reay WR, Redlich R, Reinbold CS, Repple J, Richard G, Riedel BC, Risacher SL, Rocha CS, Mota NR, Salminen L, Saremi A, Saykin AJ, Schlag F, Schmaal L, Schofield PR, Secolin R, Shapland CY, Shen L, Shin J, Shumskaya E, Sonderby IE, Sprooten E, Tansey KE, Teumer A, Thalamuthu A, Tordesillas-Gutierrez D, Turner JA, Uhlmann A, Vallerga CL, van der Meer D, van Donkelaar MMJ, van Eijk L, van Erp TGM, van Haren NEM, van Rooij D, van Tol MJ, Veldink JH, Verhoef E, Walton E, Wang M, Wang Y, Wardlaw JM, Wen W, Westlye LT, Whelan CD, Witt SH, Wittfeld K, Wolf C, Wolfers T, Wu JQ, Yasuda CL, Zaremba D, Zhang Z, Zwiers MP, Artiges E, Assareh AA, Ayesa-Arriola R, Belger A, Brandt CL, Brown GG, Cichon S, Curran JE, Davies GE, Degenhardt F, Dennis MF, Dietsche B, Djurovic S, Doherty CP, Espiritu R, Garijo D, Gil Y, Gowland PA, Green RC, Hausler AN, Heindel W, Ho BC, Hoffmann WU, Holsboer F, Homuth G, Hosten N, Jack CR Jr., Jang M, Jansen A, Kimbrel NA, Kolskar K, Koops S, Krug A, Lim KO, Luykx JJ, Mathalon DH, Mather KA, Mattay VS, Matthews S, Mayoral Van Son J, McEwen SC, Melle I, Morris DW, Mueller BA, Nauck M, Nordvik JE, Nothen MM, O’Leary DS, Opel N, Martinot MP, Pike GB, Preda A, Quinlan EB, Rasser PE, Ratnakar V, Reppermund S, Steen VM, Tooney PA, Torres FR, Veltman DJ, Voyvodic JT, Whelan R, White T, Yamamori H, Adams HHH, Bis JC, Debette S, Decarli C, Fornage M, Gudnason V, Hofer E, Ikram MA, Launer L, Longstreth WT, Lopez OL, Mazoyer B, Mosley TH, Roshchupkin GV, Satizabal CL, Schmidt R, Seshadri S, Yang Q, Alzheimer’s Disease Neuroimaging, I., Consortium, C., Consortium, E., Consortium, I., Consortium, S.Y.S., Parkinson’s Progression Markers, I., Alvim MKM, Ames D, Anderson TJ, Andreassen OA, Arias-Vasquez A, Bastin ME, Baune BT, Beckham JC, Blangero J, Boomsma DI, Brodaty H, Brunner HG, Buckner RL, Buitelaar JK, Bustillo JR, Cahn W, Cairns MJ, Calhoun V, Carr VJ, Caseras X, Caspers S, Cavalleri GL, Cendes F, Corvin A, Crespo-Facorro B, Dalrymple-Alford JC, Dannlowski U, de Geus EJC, Deary IJ, Delanty N, Depondt C, Desrivieres S, Donohoe G, Espeseth T, Fernandez G, Fisher SE, Flor H, Forstner AJ, Francks C, Franke B, Glahn DC, Gollub RL, Grabe HJ, Gruber O, Haberg AK, Hariri AR, Hartman CA, Hashimoto R, Heinz A, Henskens FA, Hillegers MHJ, Hoekstra PJ, Holmes AJ, Hong LE, Hopkins WD, Hulshoff Pol HE, Jernigan TL, Jonsson EG, Kahn RS, Kennedy MA, Kircher TTJ, Kochunov P, Kwok JBJ, Le Hellard S, Loughland CM, Martin NG, Martinot JL, McDonald C, McMahon KL, Meyer-Lindenberg A, Michie PT, Morey RA, Mowry B, Nyberg L, Oosterlaan J, Ophoff RA, Pantelis C, Paus T, Pausova Z, Penninx B, Polderman TJC, Posthuma D, Rietschel M, Roffman JL, Rowland LM, Sachdev PS, Samann PG, Schall U, Schumann G, Scott RJ, Sim K, Sisodiya SM, Smoller JW, Sommer IE, St Pourcain B, Stein DJ, Toga AW, Trollor JN, Van der Wee NJA, van t’Ent D, Volzke H, Walter H, Weber B, Weinberger DR, Wright MJ, Zhou J, Stein JL, Thompson PM, Medland SE, Enhancing NeuroImaging Genetics through Meta-Analysis Consortium -Genetics Working, G, 2020. The genetic architecture of the human cerebral cortex. Science 367 (6484). 10.1126/science.aay6690. [DOI] [Google Scholar]
- Graw S, Chappell K, Washam CL, Gies A, Bird J, Robeson MS 2nd, Byrum SD, 2021. Multi-omics data integration considerations and study design for biological systems and disease. Mol. Omics 17 (2), 170–185. 10.1039/d0mo00041h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Di L, Lu PH, Lee C, Ringman J, Apostolova LG, Chow N, Kohannim O, Cummings JL, Thompson PM, Elashoff D, Alzheimer’s Disease Neuroimaging, I, 2013. Estimating sample sizes for predementia Alzheimer’s trials based on the Alzheimer’s Disease Neuroimaging Initiative. Neurobiol. Aging 34 (1), 62–72. 10.1016/j.neurobiolaging.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh JR, Stage E, Logan PE, Iaccarino L, Joie R, Aisen PS, Eloyan A, Fagan AM, Foroud TM, Gatsonis C, Jack CR, Kramer JH, Koeppe RA, Saykin AJ, Toga AW, Vemuri P, Day GS, Graff-Radford NR, Honig LS, Jones DT, Masdeu JC, Mendez MF, Onyike CU, Rogalski EJ, Carrillo MC, Dickerson BC, Apostolova LG, 2020. Sex-associated differences in pathology burden in early-onset Alzheimer’s disease. Alzheimers Dement. 16 (S5) 10.1002/alz.046532. [DOI] [Google Scholar]
- Gurdon B, Hadad N, Telpoukhovskaia M, Yates S, Amedjkouh Puchades M, Bjaalie J, Kaczorowski C, 2020. Brain-wide spatial analysis to identify region-specific changesin cell composition associated with resilience to Alzheimer’sdisease in the AD-BXD mouse population. Alzheimers Dement. 16 10.1002/alz.047613. [DOI] [Google Scholar]
- Haas M, Stephenson D, Romero K, Gordon MF, Zach N, Geerts H, Brain Health Modeling, I, 2016. Big data to smart data in Alzheimer’s disease: Real-world examples of advanced modeling and simulation. Alzheimers Dement. 12 (9), 1022–1030. 10.1016/j.jalz.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Hampel H, O’Bryant SE, Castrillo JI, Ritchie C, Rojkova K, Broich K, Benda N, Nistico R, Frank RA, Dubois B, Escott-Price V, Lista S, 2016. Precision medicine - the golden gate for detection, treatment and prevention of Alzheimer’s disease. J. Prev. Alzheimers Dis 3 (4), 243–259. 10.14283/jpad.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Toschi N, Babiloni C, Baldacci F, Black KL, Bokde ALW, Bun RS, Cacciola F, Cavedo E, Chiesa PA, Colliot O, Coman CM, Dubois B, Duggento A, Durrleman S, Ferretti MT, George N, Genthon R, Habert MO, Herholz K, Koronyo Y, Koronyo-Hamaoui M, Lamari F, Langevin T, Lehericy S, Lorenceau J, Neri C, Nistico R, Nyasse-Messene F, Ritchie C, Rossi S, Santarnecchi E, Sporns O, Verdooner SR, Vergallo A, Villain N, Younesi E, Garaci F, Lista S, Alzheimer Precision Medicine, I, 2018. Revolution of Alzheimer precision neurology. Passageway of systems biology and neurophysiology. J. Alzheimers Dis 64 (s1), S47–S105. 10.3233/JAD-179932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Lista S, Neri C, Vergallo A, 2019a. Time for the systems-level integration of aging: resilience enhancing strategies to prevent Alzheimer’s disease. Prog. Neurobiol 181, 101662 10.1016/j.pneurobio.2019.101662. [DOI] [PubMed] [Google Scholar]
- Hampel H, Vergallo A, Perry G, Lista S, Alzheimer Precision Medicine, I, 2019b. The Alzheimer precision medicine initiative. J. Alzheimers Dis 68 (1), 1–24. 10.3233/JAD-181121. [DOI] [PubMed] [Google Scholar]
- Hampel H, Nistico R, Seyfried NT, Levey AI, Modeste E, Lemercier P, Baldacci F, Toschi N, Garaci F, Perry G, Emanuele E, Valenzuela PL, Lucia A, Urbani A, Sancesario GM, Mapstone M, Corbo M, Vergallo A, Lista S, Alzheimer Precision Medicine, I, 2021. Omics sciences for systems biology in Alzheimer’s disease: state-of-the-art of the evidence. Ageing Res. Rev 69, 101346 10.1016/j.arr.2021.101346. [DOI] [PubMed] [Google Scholar]
- Han S, Shin J, Jung H, Ryu J, Minassie H, Nho K, Koh I, Lee Y, 2021. ADAS-viewer: web-based application for integrative analysis of multi-omics data in Alzheimer’s disease. NPJ Syst. Biol. Appl 7 (1), 18. 10.1038/s41540-021-00177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297 (5580), 353–356. 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA, 1992. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256 (5054), 184–186. [DOI] [PubMed] [Google Scholar]
- Harrison JR, Bhatia S, Tan ZX, Mirza-Davies A, Benkert H, Tax CMW, Jones DK, 2020. Imaging Alzheimer’s genetic risk using diffusion MRI: a systematic review. Neuroimage Clin. 27, 102359 10.1016/j.nicl.2020.102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz M, Baldock RA, Burger A, Hashikawa T, Johnson GA, Martone M, Ng L, Lau C, Larson SD, Nissanov J, Puelles L, Ruffins S, Verbeek F, Zaslavsky I, Boline J, 2011. Digital atlasing and standardization in the mouse brain. PLoS Comput. Biol 7 (2), e1001065 10.1371/journal.pcbi.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Huisken J, 2020. Image quality guided smart rotation improves coverage in microscopy. Nat. Commun 11 (1), 150. 10.1038/s41467-019-13821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80 (19), 1778–1783. 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Mitra PP, 2012. Computational methods and challenges for large-scale circuit mapping. Curr. Opin. Neurobiol 22 (1), 162–169. 10.1016/j.conb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-de-Diego R, Tarazona S, Martinez-Mira C, Balzano-Nogueira L, FurioTari P, Pappas GJ Jr., Conesa A, 2018. PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nucleic Acids Res. 46 (W1), W503–W509. 10.1093/nar/gky466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer SE, Neuner SM, Hadad N, O’Connell KMS, Williams RW, Philip VM, Gaiteri C, Kaczorowski CC, 2020. Identifying the molecular systems that influence cognitive resilience to Alzheimer’s disease in genetically diverse mice. Learn. Mem 27 (9), 355–371. 10.1101/lm.051839.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Hassan BA, 2005. Genetics in the age of systems biology. Cell 123 (7), 1173–1174. 10.1016/j.cell.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hjornevik T, Leergaard TB, Darine D, Moldestad O, Dale AM, Willoch F, Bjaalie JG, 2007. Three-dimensional atlas system for mouse and rat brain imaging data. Front. Neuroinform 1, 4. 10.3389/neuro.11.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Buckholtz N, 2016. Accelerating medicines partnership: Alzheimer’s disease (AMP-AD) knowledge portal aids Alzheimer’s drug discovery through open data sharing. Expert Opin. Ther. Targets 20 (4), 389–391. 10.1517/14728222.2016.1135132. [DOI] [PubMed] [Google Scholar]
- Hofer E, Roshchupkin GV, Adams HHH, Knol MJ, Lin H, Li S, Zare H, Ahmad S, Armstrong NJ, Satizabal CL, Bernard M, Bis JC, Gillespie NA, Luciano M, Mishra A, Scholz M, Teumer A, Xia R, Jian X, Mosley TH, Saba Y, Pirpamer L, Seiler S, Becker JT, Carmichael O, Rotter JI, Psaty BM, Lopez OL, Amin N, van der Lee SJ, Yang Q, Himali JJ, Maillard P, Beiser AS, DeCarli C, Karama S, Lewis L, Harris M, Bastin ME, Deary IJ, Veronica Witte A, Beyer F, Loeffler M, Mather KA, Schofield PR, Thalamuthu A, Kwok JB, Wright MJ, Ames D, Trollor J, Jiang J, Brodaty H, Wen W, Vernooij MW, Hofman A, Uitterlinden AG, Niessen WJ, Wittfeld K, Bulow R, Volker U, Pausova Z, Bruce Pike G, Maingault S, Crivello F, Tzourio C, Amouyel P, Mazoyer B, Neale MC, Franz CE, Lyons MJ, Panizzon MS, Andreassen OA, Dale AM, Logue M, Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Stein JL, Thompson PM, Medland SE, Consortium, E., Sachdev PS, Kremen WS, Wardlaw JM, Villringer A, van Duijn CM, Grabe HJ, Longstreth WT Jr., Fornage M, Paus T, Debette S, Ikram MA, Schmidt H, Schmidt R, Seshadri S, 2020. Genetic correlations and genome-wide associations of cortical structure in general population samples of 22,824 adults. Nat. Commun 11 (1), 4796. 10.1038/s41467-020-18367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE, 2000. FDG PET imaging in patients with pathologically verified dementia. J. Nucl. Med 41 (11), 1920–1928. [PubMed] [Google Scholar]
- Hohman TJ, Kaczorowski CC, 2020. Modifiable lifestyle factors in Alzheimer disease: an opportunity to transform the therapeutic landscape through transdisciplinary collaboration. JAMA Neurol. 77 (10), 1207–1209. 10.1001/jamaneurol.2020.1114. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL, Alzheimer’s Disease Neuroimaging, I, 2016. Asymptomatic Alzheimer disease: Defining resilience. Neurology 87 (23), 2443–2450. 10.1212/WNL.0000000000003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang M-C, Toga AW, Jack CR, Weiner MW, Thompson PM, 2008. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage 43 (3), 458–469. 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Chaudhary K, Garmire LX, 2017. More is better: recent progress in multi-omics data integration methods. Front. Genet 8, 84. 10.3389/fgene.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhan X, Xiang S, Johnson TS, Helm B, Yu CY, Zhang J, Salama P, Rizkalla M, Han Z, Huang K, 2019. SALMON: survival analysis learning with multi-omics neural networks on breast cancer. Front. Genet 10, 166. 10.3389/fgene.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BM, Fentonm GW, Anderson JM, 1990. A quantitative histological study of early clinical and preclinical Alzheimer’s disease. Neuropathol. Appl. Neurobiol 16 (2), 111–121. 10.1111/j.1365-2990.1990.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12 (2), 207–216. 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ, 2010. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9 (1), 119–128. 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, Weigand SD, Therneau TM, Knopman DS, Gunter JL, Jones DT, Graff-Radford J, Kantarci K, Roberts RO, Mielke MM, Machulda MM, Petersen RC, 2018. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 141 (5), 1517–1528. 10.1093/brain/awy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M, 2006. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann. Neurol 59 (4), 673–681. 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Zheng H, 2017. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener 12 (1), 89. 10.1186/s13024-017-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, Fakhry A, Deng H, 2014. Integrative analysis of the connectivity and gene expression atlases in the mouse brain. NeuroImage 84, 245–253. 10.1016/j.neuroimage.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Wilkins CH, Morris JC, 2006. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch. Neurol 63 (9), 1312–1317. 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Badea A, Brandenburg J, Cofer G, Fubara B, Liu S, Nissanov J, 2010. Waxholm space: an image-based reference for coordinating mouse brain research. NeuroImage 53 (2), 365–372. 10.1016/j.neuroimage.2010.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Fox NC, Sperling RA, Klunk WE, 2012. Brain imaging in Alzheimer disease. Cold Spring Harbor Perspect. Med 2 (4) 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Ringman JM, Lee AS, Juarez KO, Mendez MF, 2012. Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J. Neurol 259 (10), 2182–2188. 10.1007/s00415-012-6481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen M, Prakash M, Zare M, Tohka J, For the Alzheimer’s Disease Neuroimaging, I, 2020. Structural brain imaging phenotypes of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) found by hierarchical clustering. Int. J. Alzheimers Dis 2020, 2142854. 10.1155/2020/2142854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow DS, McEvoy LK, Fennema-Notestine C, Hagler DJ Jr., Jennings RG, Brewer JB, Hoh CK, Dale AM, Alzheimer’s Disease Neuroimaging, I, 2010. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology 256 (3), 932–942. 10.1148/radiol.10091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CD, Darvas M, Kraemer B, Liggitt D, Sigurdson C, Ladiges W, 2016. Neuropathological assessment and validation of mouse models for Alzheimer’s disease: applying NIA-AA guidelines. Pathobiol. Aging Age Relat. Dis 6, 32397. 10.3402/pba.v6.32397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Domingo-Fernandez D, Iyappan A, Emon MA, Hofmann-Apitius M, Frohlich H, 2018. Using multi-scale genetic, neuroimaging and clinical data for predicting Alzheimer’s disease and reconstruction of relevant biological mechanisms. Sci. Rep 8 (1), 11173. 10.1038/s41598-018-29433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, 2018. The search for better animal models of Alzheimer’s disease. Nature 559 (7715), S13–S15. 10.1038/d41586-018-05722-9. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, 2005. The meaning of systems biology. Cell 121 (4), 503–504. 10.1016/j.cell.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kitano H, 2002a. Computational systems biology. Nature 420 (6912), 206–210. 10.1038/nature01254. [DOI] [PubMed] [Google Scholar]
- Kitano H, 2002b. Systems biology: a brief overview. Science 295 (5560), 1662–1664. 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Knutson KA, Deng Y, Pan W, 2020. Implicating causal brain imaging endophenotypes in Alzheimer’s disease using multivariable IWAS and GWAS summary data. NeuroImage 223, 117347. 10.1016/j.neuroimage.2020.117347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanko MA, Malhotra PA, 2018. Exploring Alzheimer’s disease subtypes at the prodromal stage. Brain 141 (12), 3285–3287. 10.1093/brain/awy282. [DOI] [PubMed] [Google Scholar]
- Kolinger GD, Vallez Garcia D, Willemsen ATM, Reesink FE, de Jong BM, Dierckx R, De Deyn PP, Boellaard R, 2021. Amyloid burden quantification depends on PET and MR image processing methodology. PLoS One 16 (3), e0248122. 10.1371/journal.pone.0248122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassowski M, Das V, Sahu SK, Misra BB, 2020. State of the field in multi-omics research: from computational needs to data mining and sharing. Front. Genet 11, 610798 10.3389/fgene.2020.610798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB, Biomarkers Consortium, P. E. T. R. P. T, 2013. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136 (Pt 7), 2228–2238. 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, Jenko KJ, Morse CL, Zoghbi SS, Pike VW, Turner RS, Innis RB, 2016. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol. Aging 44, 53–61. 10.1016/j.neurobiolaging.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Okamura N, Furumoto S, Tashiro M, Furukawa K, Maruyama M, Itoh M, Iwata R, Yanai K, Arai H, 2007. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J. Nucl. Med 48 (4), 553–561. 10.2967/jnumed.106.037556. [DOI] [PubMed] [Google Scholar]
- Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A, Bellenguez C, Frizatti A, Chouraki V, Martin ER, Sleegers K, Badarinarayan N, Jakobsdottir J, Hamilton-Nelson KL, Moreno-Grau S, Olaso R, Raybould R, Chen Y, Kuzma AB, Hiltunen M, Morgan T, Ahmad S, Vardarajan BN, Epelbaum J, Hoffmann P, Boada M, Beecham GW, Garnier JG, Harold D, Fitzpatrick AL, Valladares O, Moutet ML, Gerrish A, Smith AV, Qu L, Bacq D, Denning N, Jian X, Zhao Y, Del Zompo M, Fox NC, Choi SH, Mateo I, Hughes JT, Adams HH, Malamon J, Sanchez-Garcia F, Patel Y, Brody JA, Dombroski BA, Naranjo MCD, Daniilidou M, Eiriksdottir G, Mukherjee S, Wallon D, Uphill J, Aspelund T, Cantwell LB, Garzia F, Galimberti D, Hofer E, Butkiewicz M, Fin B, Scarpini E, Sarnowski C, Bush WS, Meslage S, Kornhuber J, White CC, Song Y, Barber RC, Engelborghs S, Sordon S, Voijnovic D, Adams PM, Vandenberghe R, Mayhaus M, Cupples LA, Albert MS, De Deyn PP, Gu W, Himali JJ, Beekly D, Squassina A, Hartmann AM, Orellana A, Blacker D, Rodriguez-Rodriguez E, Lovestone S, Garcia ME, Doody RS, Munoz-Fernadez C, Sussams R, Lin H, Fairchild TJ, Benito YA, Holmes C, Karamujic-Comic H, Frosch MP, Thonberg H,Maier W, Roshchupkin G, Ghetti B, Giedraitis V, Kawalia A, Li S, Huebinger RM, Kilander L, Moebus S, Hernandez I, Kamboh MI, Brundin R, Turton J, Yang Q, Katz MJ, Concari L, Lord J, Beiser AS, Keene CD, Helisalmi S, Kloszewska I, Kukull WA, Koivisto AM, Lynch A, Tarraga L, Larson EB, Haapasalo A, Lawlor B, Mosley TH, Cruchaga C, Graff C, Gwilliam R, Fornage M, Goate AM, Sanchez-Juan P, Kehoe PG, Amin N, Ertekin-Taner N, Berr C, Debette S, Love S, Launer LJ, Younkin SG, Dartigues JF, Corcoran C, Ikram MA, Dickson DW, Nicolas G, Campion D, Tschanz J, Schmidt H, Hakonarson H, Clarimon J, Munger R, Schmidt R, Farrer LA, Van Broeckhoven C, De Stefano AL, Jones L, Haines JL, Deleuze JF, Owen MJ, Gudnason V, Mayeux R, Escott-Price V, Psaty BM, Ramirez A, Wang LS, Ruiz A, van Duijn CM, Holmans PA, Seshadri S, Williams J, Amouyel P, Schellenberg GD, Lambert JC, Pericak-Vance MA, Alzheimer Disease Genetics, C, European Alzheimer’s Disease, I., Cohorts for, H., Aging Research in Genomic Epidemiology, C., Genetic, Environmental Risk in Ad/Defining Genetic, P., & Environmental Risk for Alzheimer’s Disease, C, 2019. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet 51 (3), 414–430. 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TC, Tian TF, Tseng YJ, 2013. 3Omics: a web-based systems biology tool for analysis, integration and visualization of human transcriptomic, proteomic and metabolomic data. BMC Syst. Biol 7, 64. 10.1186/1752-0509-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde J, Sarazin M, Bottlaender M, 2018. In vivo PET imaging of neuroinflammation in Alzheimer’s disease. J. Neural Transm. (Vienna) 125 (5), 847–867. 10.1007/s00702-017-1731-x. [DOI] [PubMed] [Google Scholar]
- Lam S, Bayraktar A, Zhang C, Turkez H, Nielsen J, Boren J, Shoaie S, Uhlen M, Mardinoglu A, 2020. A systems biology approach for studying neurodegenerative diseases. Drug Discov. Today 25 (7), 1146–1159. 10.1016/j.drudis.2020.05.010. [DOI] [PubMed] [Google Scholar]
- Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thornton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin C-F, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau M-T, Choi S-H, Reitz C, Pasquier F, Hollingworth P, Ramirez A, Hanon O, Fitzpatrick AL, Buxbaum JD, Campion D, Crane PK, Baldwin C, Becker T, Gudnason V, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fíevet N, Huentelman MJ, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuinness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Naranjo MCD, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannfelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RFAG, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JSK, Boerwinkle E, Riemenschneider M, Boada M, Hiltunen M, Martin ER, Schmidt R, Rujescu D, Wang L-S, Dartigues J-F, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45 (12), 1452–1458. 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, Pontecorvo M, Baker SL, Jagust WJ, 2014. Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur. J. Nucl. Med. Mol. Imaging 41 (7), 1398–1407. 10.1007/s00259-014-2753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley GR, 2014. Considering a new paradigm for Alzheimer’s disease research. Drug Discov. Today 19 (8), 1114–1124. 10.1016/j.drudis.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Lawrence E, Vegvari C, Ower A, Hadjichrysanthou C, De Wolf F, Anderson RM, 2017. A systematic review of longitudinal studies which measure Alzheimer’s disease biomarkers. J. Alzheimers Dis 59 (4), 1359–1379. 10.3233/JAD-170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandrou S, Petroudi S, Kyriacou PA, Reyes-Aldasoro CC, Pattichis CS, 2018. Quantitative MRI brain studies in mild cognitive impairment and Alzheimer’s disease: a methodological review. IEEE Rev. Biomed. Eng 11, 97–111. 10.1109/RBME.2018.2796598. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Masliah E, 2010. Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nat. Rev. Neurol 6 (2), 108–119. 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E, Nordberg A, 2019. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 24 (8), 1112–1134. 10.1038/s41380-018-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin F, Ferreira D, Lange C, Dyrba M, Westman E, Buchert R, Teipel SJ, Grothe MJ, Alzheimer’s Disease Neuroimaging, I., 2021. Data-driven FDG-PET subtypes of Alzheimer’s disease-related neurodegeneration. Alzheimers Res Ther 13 (1), 49. 10.1186/s13195-021-00785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JQ, Wang HF, Zhu XC, Sun FR, Tan MS, Tan CC, Jiang T, Tan L, Yu JT, Alzheimer’s Disease Neuroimaging, I, 2017. GWAS-linked loci and neuroimaging measures in Alzheimer’s disease. Mol. Neurobiol 54 (1), 146–153. 10.1007/s12035-015-9669-1. [DOI] [PubMed] [Google Scholar]
- Li XY, Men WW, Zhu H, Lei JF, Zuo FX, Wang ZJ, Zhu ZH, Bao XJ, Wang RZ, 2016. Age- and brain region-specific changes of glucose metabolic disorder, learning, and memory dysfunction in early Alzheimer’s disease assessed in APP/PS1 transgenic mice using (18)F-FDG-PET. Int. J. Mol. Sci 17 (10) 10.3390/ijms17101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Huang J, Liu W, Jin H, Li L, Zhang X, Nie B, Lin R, Tao J, Zhao S, Shan B, Chen L, 2017. Magnetic resonance spectroscopy analysis of neurochemical changes in the atrophic hippocampus of APP/PS1 transgenic mice. Behav. Brain Res 335, 26–31. 10.1016/j.bbr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Lichtenegger A, Muck M, Eugui P, Harper DJ, Augustin M, Leskovar K, Hitzenberger CK, Woehrer A, Baumann B, 2018. Assessment of pathological features in Alzheimer’s disease brain tissue with a large field-of-view visible-light optical coherence microscope. Neurophotonics 5 (3), 035002. 10.1117/1.NPh.5.3.035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista S, Khachaturian ZS, Rujescu D, Garaci F, Dubois B, Hampel H, 2016. Application of systems theory in longitudinal studies on the origin and progression of Alzheimer’s disease. Methods Mol. Biol 1303, 49–67. 10.1007/978-1-4939-2627-5_2. [DOI] [PubMed] [Google Scholar]
- Liu ET, 2005. Systems biology, integrative biology, predictive biology. Cell 121 (4), 505–506. 10.1016/j.cell.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Liu PK, Liu CH, 2011. Gene targeting MRI: nucleic acid-based imaging and applications. Methods Mol. Biol 711, 363–377. 10.1007/978-1-61737-992-5_18. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li Z, Ge Q, Lin N, Xiong M, 2019. Deep feature selection and causal analysis of Alzheimer’s disease. Front. Neurosci 13, 1198. 10.3389/fnins.2019.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Unsal HS, Tao Y, Zhang N, 2020. Automatic brain extraction for rodent MRI images. Neuroinformatics 18 (3), 395–406. 10.1007/s12021-020-09453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N, 2017. Dementia prevention, intervention, and care. Lancet 390 (10113), 2673–2734. 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N, 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396 (10248), 413–446. 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock EF, Hoadley KA, Marron JS, Nobel AB, 2013. Joint and individual variation explained (jive) for integrated analysis of multiple data types. Ann. Appl. Stat 7 (1), 523–542. 10.1214/12-AOAS597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A, 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412 (6843), 150–157. 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Ma Y, Klein HU, De Jager PL, 2020. Considerations for integrative multi-omic approaches to explore Alzheimer’s disease mechanisms. Brain Pathol. 30 (5), 984–991. 10.1111/bpa.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, Rabinovici GD, Jagust WJ, Alzheimer’s Disease Neuroimaging, I, 2017. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. NeuroImage 157, 448–463. 10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR, 2003. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 61 (4), 500–506. 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahecic D, Gambarotto D, Douglass KM, Fortun D, Banterle N, Ibrahim KA, Le Guennec M, Gonczy P, Hamel V, Guichard P, Manley S, 2020. Homogeneous multifocal excitation for high-throughput super-resolution imaging. Nat. Methods 17 (7), 726–733. 10.1038/s41592-020-0859-z. [DOI] [PubMed] [Google Scholar]
- Maheswaran S, Barjat H, Rueckert D, Bate ST, Howlett DR, Tilling L, Smart SC, Pohlmann A, Richardson JC, Hartkens T, Hill DL, Upton N, Hajnal JV, James MF, 2009. Longitudinal regional brain volume changes quantified in normal aging and Alzheimer’s APP × PS1 mice using MRI. Brain Res. 1270, 19–32. 10.1016/j.brainres.2009.02.045. [DOI] [PubMed] [Google Scholar]
- Mannheim JG, Kara F, Doorduin J, Fuchs K, Reischl G, Liang S, Verhoye M, Gremse F, Mezzanotte L, Huisman MC, 2018. Standardization of small animal imaging-current status and future prospects. Mol. Imaging Biol 20 (5), 716–731. 10.1007/s11307-017-1126-2. [DOI] [PubMed] [Google Scholar]
- Marcus C, Mena E, Subramaniam RM, 2014. Brain PET in the diagnosis of Alzheimer’s disease. Clin. Nucl. Med 39 (10), e413–e426. 10.1097/RLU.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, 1997. Neuropathological criteria for the diagnosis of Alzheimer’s disease. Neurobiol. Aging 18 (4 Suppl), S13–S19. 10.1016/s0197-4580(97)00064-x. [DOI] [PubMed] [Google Scholar]
- Márquez F, Yassa MA, 2019. Neuroimaging biomarkers for Alzheimer’s disease. Mol. Neurodegener 14 (1), 21. 10.1186/s13024-019-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba N, Stein JL, 2021. From base pair to brain. Nat. Neurosci 24 (5), 619–621. 10.1038/s41593-021-00852-2. [DOI] [PubMed] [Google Scholar]
- Mayo CD, Garcia-Barrera MA, Mazerolle EL, Ritchie LJ, Fisk JD, Gawryluk JR, 2019. Relationship between DTI metrics and cognitive function in Alzheimer’s disease. Front. Aging Neurosci 10 10.3389/fnagi.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, McEvoy CT, 2019. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep 8 (2), 53–65. 10.1007/s13668-019-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AP, Chen MK, O’Dell RS, Naganawa M, Toyonaga T, Godek TA, Harris JE, Bartlett HH, Zhao W, Nabulsi NB, Wyk BCV, Varma P, Arnsten AFT, Huang Y, Carson RE, van Dyck CH, 2020. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 16 (7), 974–982. 10.1002/alz.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Narayanan B, Liu J, Perrone-Bizzozero NI, Stevens MC, Calhoun VD, Glahn DC, Shen L, Risacher SL, Saykin AJ, Pearlson GD, 2012. A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer’s disease in the ADNI cohort. NeuroImage 60 (3), 1608–1621. 10.1016/j.neuroimage.2011.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, 2017. Early-onset Alzheimer disease. Neurol. Clin 35 (2), 263–281. 10.1016/j.ncl.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Li J, Zhang Q, Chen F, Bian C, Yao X, Yan J, Xu Z, Risacher SL, Saykin AJ, Liang H, Shen L, Alzheimer’s Disease Neuroimaging, I, 2020. Multivariate genome wide association and network analysis of subcortical imaging phenotypes in Alzheimer’s disease. BMC Genomics 21 (Suppl. 11), 896. 10.1186/s12864-020-07282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA, 2014. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol 6, 37–48. 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei N, Tang SP, Ashworth S, Coello C, Plisson C, Passchier J, Selvaraj V, Tyacke RJ, Nutt DJ, Sastre M, 2016. In vivo imaging of microglial activation by positron emission tomography with [(11)C]PBR28 in the 5XFAD model of Alzheimer’s disease. Glia 64 (6), 993–1006. 10.1002/glia.22978. [DOI] [PubMed] [Google Scholar]
- Misra BB, Langefeld CD, Olivier M, Cox LA, 2018. Integrated omics: tools, advances, and future approaches. J. Mol. Endocrinol 10.1530/JME-18-0055. [DOI] [PubMed] [Google Scholar]
- Mitra PP, 2014. The circuit architecture of whole brains at the mesoscopic scale. Neuron 83 (6), 1273–1283. 10.1016/j.neuron.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty R, Martensson G, Poulakis K, Muehlboeck JS, Rodriguez-Vieitez E, Chiotis K, Grothe MJ, Nordberg A, Ferreira D, Westman E, 2020. Comparison of subtyping methods for neuroimaging studies in Alzheimer’s disease: a call for harmonization. Brain Commun. 2 (2), fcaa192. 10.1093/braincomms/fcaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody JN, Valerio KE, Hasselbach M, Prieto S, Logue M, Hayes SM, Hayes JP, Alzheimer’s Disease Neuroimaging, I, 2021. Body mass index and polygenic risk for Alzheimer’s disease predict conversion to Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci 10.1093/gerona/glab117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Murphy GG, Cazares VA, 2020. Turning strains into strengths for understanding psychiatric disorders. Mol. Psychiatry 25 (12), 3164–3177. 10.1038/s41380-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli S, Bauckneht M, 2018. Amyloid PET imaging: standardization and integration with other Alzheimer’s disease biomarkers. Methods Mol. Biol 1750, 203–212. 10.1007/978-1-4939-7704-8_13. [DOI] [PubMed] [Google Scholar]
- Mosconi L, 2005. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 32 (4), 486–510. 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Mroczek M, Desouky A, Sirry W, 2021. Imaging transcriptomics in neurodegenerative diseases. J. Neuroimaging 31 (2), 244–250. 10.1111/jon.12827. [DOI] [PubMed] [Google Scholar]
- Mueller A, Bullich S, Barret O, Madonia J, Berndt M, Papin C, Perrotin A, Koglin N, Kroth H, Pfeifer A, Tamagnan G, Seibyl JP, Marek K, De Santi S, Dinkelborg LM, Stephens AW, 2020. Tau PET imaging with (18)F-PI-2620 in patients with Alzheimer disease and healthy controls: a first-in-humans study. J. Nucl. Med 61 (6), 911–919. 10.2967/jnumed.119.236224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L, 2005. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin. N. Am 15 (4), 869–877 xi–xii. 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Castaneda R, Zingg B, Matho KS, Chen X, Wang Q, Foster NN, Li A, Narasimhan A, Hirokawa KE, Huo B, Bannerjee S, Korobkova L, Park CS, Park YG, Bienkowski MS, Chon U, Wheeler DW, Li X, Wang Y, Naeemi M, Xie P, Liu L, Kelly K, An X, Attili SM, Bowman I, Bludova A, Cetin A, Ding L, Drewes R, D’Orazi F, Elowsky C, Fischer S, Galbavy W, Gao L, Gillis J, Groblewski PA, Gou L, Hahn JD, Hatfield JT, Hintiryan H, Huang JJ, Kondo H, Kuang X, Lesnar P, Li X, Li Y, Lin M, Lo D, Mizrachi J, Mok S, Nicovich PR, Palaniswamy R, Palmer J, Qi X, Shen E, Sun YC, Tao HW, Wakemen W, Wang Y, Yao S, Yuan J, Zhan H, Zhu M, Ng L, Zhang LI, Lim BK, Hawrylycz M, Gong H, Gee JC, Kim Y, Chung K, Yang XW, Peng H, Luo Q, Mitra PP, Zador AM, Zeng H, Ascoli GA, Josh Huang Z, Osten P, Harris JA, Dong HW, 2021. Cellular anatomy of the mouse primary motor cortex. Nature 598 (7879), 159–166. 10.1038/s41586-021-03970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW, 2011. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10 (9), 785–796. 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderali EK, Ratcliffe SH, Dale MC, 2009. Review: obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am. J. Alzheimer’s Disease Other Dementiasr 24 (6), 445–449. 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativio R, Lan Y, Donahue G, Sidoli S, Berson A, Srinivasan AR, Shcherbakova O, Amlie-Wolf A, Nie J, Cui X, He C, Wang LS, Garcia BA, Trojanowski JQ, Bonini NM, Berger SL, 2020. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat. Genet 52 (10), 1024–1035. 10.1038/s41588-020-0696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Croteau DL, Jurek A, Andrusivova Z, Yang B, Wang Y, Ogedegbe B, Riaz T, Stoen M, Desler C, Rasmussen LJ, Tonjum T, Galas MC, Lundeberg J, Bohr VA, 2020. Spatial transcriptomics reveals genes associated with dysregulated mitochondrial functions and stress signaling in alzheimer disease. iScience 23 (10), 101556. 10.1016/j.isci.2020.101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Yashin AI, Kulminski AM, 2019. Genome-wide analysis of genetic predisposition to Alzheimer’s disease and related sex disparities. Alzheimers Res. Ther 11 (1), 5. 10.1186/s13195-018-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff EP, 2019. Animal models of Alzheimer’s disease embrace diversity. Lab Anim (NY) 48 (9), 255–259. 10.1038/s41684-019-0377-8. [DOI] [PubMed] [Google Scholar]
- Neff RA, Wang M, Vatansever S, Guo L, Ming C, Wang Q, Wang E, Horgusluoglu-Moloch E, Song W-M, Li A, Castranio EL, Tcw J, Ho L, Goate A, Fossati V, Noggle S, Gandy S, Ehrlich ME, Katsel P, Schadt E, Cai D, Brennand KJ, Haroutunian V, Zhang B, 2021. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci. Adv 7 (2), eabb5398. 10.1126/sciadv.abb5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash S, Xie S, Davatzikos C, Clark CM, Trojanowski JQ, Shaw LM, Wolk DA, Arnold SE, 2013. Cognitive and functional resilience despite molecular evidence of Alzheimer’s disease pathology. Alzheimers Dement. 9 (3), e89–e95. 10.1016/j.jalz.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Garfinkel BP, Wilmott LA, Ignatowska-Jankowska BM, Citri A, Orly J, Lu L, Overall RW, Mulligan MK, Kempermann G, Williams RW, O’Connell KM, Kaczorowski CC, 2016. Systems genetics identifies Hp1bp3 as a novel modulator of cognitive aging. Neurobiol. Aging 46, 58–67. 10.1016/j.neurobiolaging.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Hohman TJ, Richholt R, Bennett DA, Schneider JA, De Jager PL, Huentelman MJ, O’Connell KMS, Kaczorowski CC, 2017a. Systems genetics identifies modifiers of Alzheimer’s disease risk and resilience. bioRxiv. 10.1101/225714. [DOI] [Google Scholar]
- Neuner SM, Wilmott LA, Hoffmann BR, Mozhui K, Kaczorowski CC, 2017b. Hippocampal proteomics defines pathways associated with memory decline and resilience in normal aging and Alzheimer’s disease mouse models. Behav. Brain Res 322 (Pt B), 288–298. 10.1016/j.bbr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Heuer SE, Huentelman MJ, O’Connell KMS, Kaczorowski CC, 2019a. Harnessing genetic complexity to enhance translatability of Alzheimer’s disease mouse models: a path toward precision medicine. Neuron 101 (3), 399–411 e395. 10.1016/j.neuron.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Heuer SE, Zhang JG, Philip VM, Kaczorowski CC, 2019b. Identification of pre-symptomatic gene signatures that predict resilience to cognitive decline in the genetically diverse AD-BXD model. Front. Genet 10, 35. 10.3389/fgene.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Tcw J, Goate AM, 2020. Genetic architecture of Alzheimer’s disease. Neurobiol. Dis 143, 104976 10.1016/j.nbd.2020.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Bernard A, Lau C, Overly CC, Dong HW, Kuan C, Pathak S, Sunkin SM, Dang C, Bohland JW, Bokil H, Mitra PP, Puelles L, Hohmann J, Anderson DJ, Lein ES, Jones AR, Hawrylycz M, 2009. An anatomic gene expression atlas of the adult mouse brain. Nat. Neurosci 12 (3), 356–362. 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- Nicora G, Vitali F, Dagliati A, Geifman N, Bellazzi R, 2020. Integrated multi-omics analyses in oncology: a review of machine learning methods and tools. Front. Oncol 10, 1030. 10.3389/fonc.2020.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedworok CJ, Brown AP, Jorge Cardoso M, Osten P, Ourselin S, Modat M, Margrie TW, 2016. aMAP is a validated pipeline for registration and segmentation of high-resolution mouse brain data. Nat. Commun 7, 11879. 10.1038/ncomms11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC, 2009. Neuropathologic studies of the baltimore longitudinal study of aging (BLSA). J. Alzheimers Dis 18 (3), 665–675. 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell KMS, Ouellette AR, Neuner SM, Dunn AR, Kaczorowski CC, 2019. Genetic background modifies CNS-mediated sensorimotor decline in the AD-BXD mouse model of genetic diversity in Alzheimer’s disease. Genes Brain Behav. 18 (8), e12603 10.1111/gbb.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell RS, Mecca AP, Chen MK, Naganawa M, Toyonaga T, Lu Y, Godek TA, Harris JE, Bartlett HH, Banks ER, Kominek VL, Zhao W, Nabulsi NB, Ropchan J, Ye Y, Vander Wyk BC, Huang Y, Arnsten AFT, Carson RE, van Dyck CH, 2021. Association of Abeta deposition and regional synaptic density in early Alzheimer’s disease: a PET imaging study with [(11)C]UCB-J. Alzheimers Res. Ther 13 (1), 11. 10.1186/s13195-020-00742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori E, DeKosky ST, Febo M, Colon-Perez L, Chakrabarty P, Duara R, Adjouadi M, Golde TE, Vaillancourt DE, 2019. Free-water imaging of the hippocampus is a sensitive marker of Alzheimer’s disease. NeuroImage: Clin. 24, 101985 10.1016/j.nicl.2019.101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P, 1990. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med 14 (1), 68–78. 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H, 2014. A mesoscale connectome of the mouse brain. Nature 508 (7495), 207–214. 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Akhter K, Mielke M, Ceritoglu C, Zhang J, Jiang H, Li X, Younes L, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Mori S, 2011. Multi-modal MRI analysis with disease-specific spatial filtering: initial testing to predict mild cognitive impairment patients who convert to Alzheimer’s disease. Front. Neurol 2, 54. 10.3389/fneur.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onos KD, Sukoff Rizzo SJ, Howell GR, Sasner M, 2016. Toward more predictive genetic mouse models of Alzheimer’s disease. Brain Res. Bull 122, 1–11. 10.1016/j.brainresbull.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onos KD, Uyar A, Keezer KJ, Jackson HM, Preuss C, Acklin CJ, O’Rourke R, Buchanan R, Cossette TL, Sukoff Rizzo SJ, Soto I, Carter GW, Howell GR, 2019. Enhancing face validity of mouse models of Alzheimer’s disease with natural genetic variation. PLoS Genet. 15 (5), e1008155 10.1371/journal.pgen.1008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DR, Kuntner C, Berr S, Stout D, 2017. Guidance for efficient small animal imaging quality control. Mol. Imaging Biol 19 (4), 485–498. 10.1007/s11307-016-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Tolboom N, Foster-Dingley JC, Adriaanse SF, Boellaard R, Yaqub M, Windhorst AD, Barkhof F, Lammertsma AA, Scheltens P, van der Flier WM, van Berckel BN, 2012. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur. J. Nucl. Med. Mol. Imaging 39 (6), 990–1000. 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Lyoo CH, Sudre CH, van Westen D, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S, Westman E, Tsai R, Kramer J, Boxer AL, Gorno-Tempini ML, La Joie R, Miller BL, Rabinovici GD, Hansson O, 2020. Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease. Alzheimers Dement. 16 (2), 335–344. 10.1016/j.jalz.2019.08.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Margrie TW, 2013. Mapping brain circuitry with a light microscope. Nat. Methods 10 (6), 515–523. 10.1038/nmeth.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K, Oishi N, Ito K, Fukuyama H, Group, S.J.S., Alzheimer’s Disease Neuroimaging, I, 2015. Effects of imaging modalities, brain atlases and feature selection on prediction of Alzheimer’s disease. J. Neurosci. Methods 256, 168–183. 10.1016/j.jneumeth.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martínez-Lage P, 2014. Diet, cognition, and Alzheimer’s disease: food for thought. Eur. J. Nutr 53 (1), 1–23. 10.1007/s00394-013-0561-3. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Chen K, Yao L, Wu X, Zhang J, Li K, Jin Z, Guo X, 2015. Independent component analysis-based identification of covariance patterns of microstructural white matter damage in Alzheimer’s disease. PLoS One 10 (3), e0119714. 10.1371/journal.pone.0119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA, 2018. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 136 (6), 887–900. 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Damiano M, Galbusera A, Tsaftaris SA, Gozzi A, 2016. Semi-automated registration-based anatomical labelling, voxel based morphometry and cortical thickness mapping of the mouse brain. J. Neurosci. Methods 267, 62–73. 10.1016/j.jneumeth.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Pallast N, Diedenhofen M, Blaschke S, Wieters F, Wiedermann D, Hoehn M, Fink GR, Aswendt M, 2019. Processing pipeline for atlas-based imaging data analysis of structural and functional mouse brain MRI (AIDAmri). Front. Neuroinform 13, 42. 10.3389/fninf.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Na HK, Kim S, Kim H, Kim HJ, Seo SW, Na DL, Han CE, Seong JK, Alzheimer’s Disease Neuroimaging, I, 2017. Robust Identification of Alzheimer’s Disease subtypes based on cortical atrophy patterns. Sci. Rep 7, 43270. 10.1038/srep43270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Gatz M, Berg S, Johansson B, 2004. How heritable is Alzheimer’s disease late in life? Findings from Swedish twins. Ann. Neurol 55 (2), 180–185. 10.1002/ana.10999. [DOI] [PubMed] [Google Scholar]
- Peirce JL, Lu L, Gu J, Silver LM, Williams RW, 2004. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 5, 7. 10.1186/1471-2156-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenova AA, Raj T, Goate AM, 2018. Untangling genetic risk for Alzheimer’s disease. Biol. Psychiatry 83 (4), 300–310. 10.1016/j.biopsych.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB, 2016. Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev 30, 25–48. 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Plant C, Teipel SJ, Oswald A, Böhm C, Meindl T, Mourao-Miranda J, Bokde AW, Hampel H, Ewers M, 2010. Automated detection of brain atrophy patterns based on MRI for the prediction of Alzheimer’s disease. NeuroImage 50 (1), 162–174. 10.1016/j.neuroimage.2009.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, McKeel DW Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC, 2009. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging 30 (7), 1026–1036. 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV, 2010. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 67 (6), 505–512. 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Prokopenko D, Hecker J, Kirchner R, Chapman BA, Hoffman O, Mullin K, Hide W, Bertram L, Laird N, DeMeo DL, Lange C, Tanzi RE, 2020. Identification of novel Alzheimer’s disease loci using sex-specific family-based association analysis of whole-genome sequence data. Sci. Rep 10 (1), 5029. 10.1038/s41598-020-61883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räihä I, Rajala T, Sourander L, Kaprio J, Koskenvuo M, 1996. Alzheimer’s disease in finnish twins. Lancet 347 (9001), 573–578. 10.1016/s0140-6736(96)91272-6. [DOI] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Shen L, McDonald BC, Yoder KK, Hutchins GD, West JD, Tallman EF, Gao S, Foroud TM, Farlow MR, De Jager PL, Bennett DA, Aisen PS, Petersen RC, Jack CR Jr., Toga AW, Green RC, Jagust WJ, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging, I, 2015. GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain 138 (Pt 10), 3076–3088. 10.1093/brain/awv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport N, Shamir R, 2019. NEMO: cancer subtyping by integration of partial multi-omic data. Bioinformatics 35 (18), 3348–3356. 10.1093/bioinformatics/btz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Jagust WJ, 2012. Brain imaging in the study of Alzheimer’s disease. NeuroImage 61 (2), 505–516. 10.1016/j.neuroimage.2011.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, 2012. Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. Int. J. Alzheimers Dis 2012, e369808 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Rogaeva E, Beecham GW, 2020. Late-onset vs nonmendelian early-onset Alzheimer disease. Neurol. Genet 6 (5) 10.1212/NXG.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, Wang VX, Tang CY, Olsen O, Dulac C, Osten P, Tessier-Lavigne M, 2016. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165 (7), 1789–1802. 10.1016/j.cell.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Models: Alzheimer’s Disease, 2021. Retrieved May 28 from. https://www.alzforum.org/research-models/alzheimers-disease.
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Lemaitre H, Mann KF, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Schumann G, Hawrylycz M, Poline JB, Greicius MD, Consortium, I, 2015. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science 348 (6240), 1241–1244. 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, Whitwell JL, Jenkins L, Black RS, Grundman M, Fox NC, 2008. Volumetric MRI and cognitive measures in Alzheimer disease: comparison of markers of progression. J. Neurol 255 (4), 567–574. 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Wong DF, Wolk DA, Leinonen V, Arnold SE, Buckley C, Smith A, McLain R, Sherwin PF, Farrar G, Kailajärvi M, Grachev ID, 2012. [(18)F] Flutemetamol PET imaging and cortical biopsy histopathology for fibrillar amyloid β detection in living subjects with normal pressure hydrocephalus: pooled analysis of four studies. Acta Neuropathol 124 (6), 833–845. 10.1007/s00401-012-1051-z. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Anderson WH, Charil A, Castelluccio PF, Shcherbinin S, Saykin AJ, Schwarz AJ, Alzheimer’s Disease Neuroimaging, I, 2017. Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89 (21), 2176–2186. 10.1212/WNL.0000000000004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohart F, Eslami A, Matigian N, Bougeard S, Le Cao KA, 2017a. MINT: a multivariate integrative method to identify reproducible molecular signatures across independent experiments and platforms. BMC Bioinform. 18 (1), 128. 10.1186/s12859-017-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohart F, Gautier B, Singh A, Le Cao KA, 2017b. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol 13 (11), e1005752 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C, Bussey TJ, Saksida LM, 2013. Paying more attention to attention: towards more comprehensive cognitive translation using mouse models of Alzheimer’s disease. Brain Res. Bull 92, 49–55. 10.1016/j.brainresbull.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P, 2000. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am. J. Neuroradiol 21 (10), 1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Rosario D, Boren J, Uhlen M, Proctor G, Aarsland D, Mardinoglu A, Shoaie S, 2020. Systems biology approaches to understand the host-microbiome interactions in neurodegenerative diseases. Front. Neurosci 14, 716. 10.3389/fnins.2020.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD, 1996. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu. Rev. Med 47 (1), 387–400. 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, Tochon Danguy H, Chan G, Berlangieri SU, Jones G, Dickinson-Rowe KL, Kung HP, Zhang W, Kung MP, Skovronsky D, Dyrks T, Holl G, Krause S, Friebe M, Lehman L, Lindemann S, Dinkelborg LM, Masters CL, Villemagne VL, 2008. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94–9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 7 (2), 129–135. 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- Roy DS, Park Y-G, Ogawa SK, Cho JH, Choi H, Kamensky L, Martin J, Chung K, Tonegawa S, 2019. Brain-wide mapping of contextual fear memory engram ensembles supports the dispersed engram complex hypothesis. bioRxiv. 10.1101/668483. [DOI] [Google Scholar]
- Royer LA, Lemon WC, Chhetri RK, Wan Y, Coleman M, Myers EW, Keller PJ, 2016. Adaptive light-sheet microscopy for long-term, high-resolution imaging in living organisms. Nat. Biotechnol 34 (12), 1267–1278. 10.1038/nbt.3708. [DOI] [PubMed] [Google Scholar]
- Rueda AD, Lau KM, Saito N, Harvey D, Risacher SL, Aisen PS, Petersen RC, Saykin AJ, Farias ST, Alzheimer’s Disease Neuroimaging, I, 2015. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement. 11 (9), 1080–1089. 10.1016/j.jalz.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JBS, Lopera F, Martins R, Masters CL, Mayeux RP, McDade E, Moreno S, Reiman EM, Ringman JM, Salloway S, Schofield PR, Sperling R, Tariot PN, Xiong C, Morris JC, Bateman RJ, Network, A. T. D. I. A, 2014. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology 83 (3), 253–260. 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara N, Perez PD, Lin WL, Dickson DW, Ren Y, Zeng H, Lewis J, Febo M, 2014. Age-related decline in white matter integrity in a mouse model of tauopathy: an in vivo diffusion tensor magnetic resonance imaging study. Neurobiol. Aging 35 (6), 1364–1374. 10.1016/j.neurobiolaging.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg G, Stewart W, Smialek J, Troncoso JC, 2001. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol. Aging 22 (2), 169–175. 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Scelsi MA, Khan RR, Lorenzi M, Christopher L, Greicius MD, Schott JM, Ourselin S, Altmann A, 2018. Genetic study of multimodal imaging Alzheimer’s disease progression score implicates novel loci. Brain: A J. Neurol 141 (7), 2167–2180. 10.1093/brain/awy141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, 2009. Imaging in Alzheimer’s disease. Dialogues Clin. Neurosci 11 (2), 191–199. 10.31887/DCNS.2009.11.2/pscheltens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M, 2014. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J. Intern. Med 275 (3), 251–283. 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Maass A, Mattsson N, Ashton NJ, Blennow K, Zetterberg H, Jagust W, 2019. Biomarkers for tau pathology. Mol. Cell. Neurosci 97, 18–33. 10.1016/j.mcn.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M, Weiner RL, Dumitrescu L, Hohman TJ, 2021. Protective genes and pathways in Alzheimer’s disease: moving towards precision interventions. Mol. Neurodegener 16 (1), 29. 10.1186/s13024-021-00452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, Foroud T, Pankratz N, Moore JH, Sloan CD, Huentelman MJ, Craig DW, Dechairo BM, Potkin SG, Jack CR Jr., Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging, I, 2010. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: a study of the ADNI cohort. NeuroImage 53 (3), 1051–1063. 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Ma S, Vemuri P, Simon G, Alzheimer’s Disease Neuroimaging, I, 2020. Challenges and opportunities with causal discovery algorithms: application to Alzheimer’s pathophysiology. Sci. Rep 10 (1), 2975. 10.1038/s41598-020-59669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE, Schapiro MB, Jagust WJ, Hoffman JM, Welsh-Bohmer KA, Alavi A, Clark CM, Salmon E, de Leon MJ, Mielke R, Cummings JL, Kowell AP, Gambhir SS, Hoh CK, Phelps ME, 2001. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA 286 (17), 2120–2127. 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- Simpson S, Chen Y, Wellmeyer E, Smith LC, Aragon Montes B, George O, Kimbrough A, 2021. The hidden brain: uncovering previously overlooked brain regions by employing novel preclinical unbiased network approaches. Front. Syst. Neurosci 15, 595507 10.3389/fnsys.2021.595507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Shannon CP, Gautier B, Rohart F, Vacher M, Tebbutt SJ, Le Cao KA, 2019. DIABLO: an integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics 35 (17), 3055–3062. 10.1093/bioinformatics/bty1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO, 2014. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis 39 (2), 271–282. 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, 2002. Imaging the progression of Alzheimer pathology through the brain. Proc. Natl. Acad. Sci. U. S. A 99 (7), 4135–4137. 10.1073/pnas.082107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Douaud G, Chen W, Hanayik T, Alfaro-Almagro F, Sharp K, Elliott LT, 2021. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci 24 (5), 737–745. 10.1038/s41593-021-00826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, Jack CR, Kaye J, Lim K, Larson EB, White L, Montine TJ, 2011. Ecology of the aging human brain. Arch. Neurol 68 (8), 1049–1056. 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, Laviolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA, 2010. Functional alterations in memory networks in early Alzheimer’s disease. NeuroMolecular Med. 12 (1), 27–43. 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber G, Simmons A, Muehlboeck J-S, Mecocci P, Vellas B, Tsolaki M, Kłoszewska I, Soininen H, Spenger C, Lovestone S, Wahlund L-O, Westman E, 2013. An MRI-based index to measure the severity of Alzheimer’s disease-like structural pattern in subjects with mild cognitive impairment. J. Intern. Med 273 (4), 396–409. 10.1111/joim.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, The Reserve, R., Protective Factors, P. I. A. E. D, Conceptual Frameworks, W, 2020. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 16 (9), 1305–1311. 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD, 1993a. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A 90 (5), 1977–1981. 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD, 1993b. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoformspecific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A 90 (17), 8098–8102. 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studies Involving BXD RI Panel, 2021. Retrieved May 28 from. https://phenome.jax.org/panels/BXD.
- Subramanian I, Verma S, Kumar S, Jere A, Anamika K, 2020. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights 14. 10.1177/1177932219899051, 1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum S, Misrani A, Yang L, 2020. Exploiting common aspects of obesity and Alzheimer’s disease. Front. Hum. Neurosci 14 10.3389/fnhum.2020.602360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wu D, Gu L-H, Nie B-B, Qi X-Y, Wang Y-J, Wu F-F, Li X-L, Bai F, Chen X-C, Xu L, Ren Q-G, Zhang Z-J, 2016. Spatial learning and memory impairments are associated with increased neuronal activity in 5XFAD mouse as measured by manganese-enhanced magnetic resonance imaging. Oncotarget 7 (36), 57556–57570. 10.18632/oncotarget.11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, 2012. The genetics of Alzheimer disease. Cold Spring Harbor Perspect. Med 2 (10) 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Greenlund SF, McGuire LC, Lu H, Croft JB, 2017. Deaths from Alzheimer’s Disease — United States, 1999–2014. Morb. Mortal. Wkly Rep 66 (20), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S, Drzezga A, Grothe MJ, Barthel H, Chetelat G, Schuff N, Skudlarski P, Cavedo E, Frisoni GB, Hoffmann W, Thyrian JR, Fox C, Minoshima S, Sabri O, Fellgiebel A, 2015. Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol. 14 (10), 1037–1053. 10.1016/S1474-4422(15)00093-9. [DOI] [PubMed] [Google Scholar]
- Ten Kate M, Dicks E, Visser PJ, van der Flier WM, Teunissen CE, Barkhof F, Scheltens P, Tijms BM, Alzheimer’s Disease Neuroimaging, I, 2018. Atrophy subtypes in prodromal Alzheimer’s disease are associated with cognitive decline. Brain 141 (12), 3443–3456. 10.1093/brain/awy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Top 10 Causes of Death, 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Toepper M, 2017. Dissociating normal aging from Alzheimer’s disease: a view from cognitive neuroscience. J. Alzheimers Dis 57 (2), 331–352. 10.3233/JAD-161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Ceyźeriat K, Garibotto V, Millet P, 2020. In vivo TSPO signal and neuroinflammation in Alzheimer’s disease. Cells 9 (9), 1941. 10.3390/cells9091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Wang Y, Woodbury-Smith M, 2019. Artificial intelligence for precision medicine in neurodevelopmental disorders. NPJ Digit. Med 2, 112. 10.1038/s41746-019-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Jack CR, 2010. Role of structural MRI in Alzheimer’s disease. Alzheimers Res. Ther 2 (4), 23. 10.1186/alzrt47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Jones DT, Jack CR Jr., 2012. Resting state functional MRI in Alzheimer’s Disease. Alzheimers Res. Ther 4 (1), 2. 10.1186/alzrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeff NPLG, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung M-P, Houle S, 2004. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am. J. Geriatr. Psychiatry 12 (6), 584–595. 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- Vetere G, Kenney JW, Tran LM, Xia F, Steadman PE, Parkinson J, Josselyn SA, Frankland PW, 2017. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94 (2), 363–374 e364. 10.1016/j.neuron.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC, 2011. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann. Neurol 69 (1), 181–192. 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JW, Young AL, Oxtoby NP, Smith R, Ossenkoppele R, Strandberg OT, La Joie R, Aksman LM, Grothe MJ, Iturria-Medina Y, Pontecorvo MJ, Devous MD, Rabinovici GD, Alexander DC, Lyoo CH, Evans AC, Hansson O, 2021. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med 1–11 10.1038/s41591-021-01309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Chamier L, Laine RF, Jukkala J, Spahn C, Krentzel D, Nehme E, Lerche M, Hernandez-Perez S, Mattila PK, Karinou E, Holden S, Solak AC, Krull A, Buchholz TO, Jones ML, Royer LA, Leterrier C, Shechtman Y, Jug F, Heilemann M, Jacquemet G, Henriques R, 2021. Democratising deep learning for microscopy with ZeroCostDL4Mic. Nat. Commun 12 (1), 2276. 10.1038/s41467-021-22518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachinger C, Nho K, Saykin AJ, Reuter M, Rieckmann A, Alzheimer’s Disease Neuroimaging, I, 2018. A longitudinal imaging genetics study of neuroanatomical asymmetry in Alzheimer’s disease. Biol. Psychiatry 84 (7), 522–530. 10.1016/j.biopsych.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Herskowitz JH, 2020. Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist. 10.1177/1073858420945964, 1073858420945964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YW, Al-Ouran R, Mangleburg CG, Perumal TM, Lee TV, Allison K, Swarup V, Funk CC, Gaiteri C, Allen M, Wang M, Neuner SM, Kaczorowski CC, Philip VM, Howell GR, Martini-Stoica H, Zheng H, Mei H, Zhong X, Kim JW, Dawson VL, Dawson TM, Pao PC, Tsai LH, Haure-Mirande JV, Ehrlich ME, Chakrabarty P, Levites Y, Wang X, Dammer EB, Srivastava G, Mukherjee S, Sieberts SK, Omberg L, Dang KD, Eddy JA, Snyder P, Chae Y, Amberkar S, Wei W, Hide W, Preuss C, Ergun A, Ebert PJ, Airey DC, Mostafavi S, Yu L, Klein HU, Accelerating Medicines Partnership-Alzheimer’s Disease, C, Carter GW, Collier DA, Golde TE, Levey AI, Bennett DA, Estrada K, Townsend TM, Zhang B, Schadt E, De Jager PL, Price ND, Ertekin-Taner N, Liu Z, Shulman JM, Mangravite LM, Logsdon BA, 2020. Meta-Analysis of the Alzheimer’s Disease Human Brain Transcriptome and Functional Dissection in Mouse Models. Cell Rep. 32 (2), 107908 10.1016/j.celrep.2020.107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Mezlini AM, Demir F, Fiume M, Tu Z, Brudno M, Haibe-Kains B, Goldenberg A, 2014. Similarity network fusion for aggregating data types on a genomic scale. Nat. Methods 11 (3), 333–337. 10.1038/nmeth.2810. [DOI] [PubMed] [Google Scholar]
- Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, McConathy J, Cairns NJ, Fagan AM, Morris JC, Ances BM, 2016a. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol. 73 (9), 1070–1077. 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Beckmann ND, Roussos P, Wang E, Zhou X, Wang Q, Ming C, Neff R, Ma W, Fullard JF, Hauberg ME, Bendl J, Peters MA, Logsdon B, Wang P, Mahajan M, Mangravite LM, Dammer EB, Duong DM, Lah JJ, Seyfried NT, Levey AI, Buxbaum JD, Ehrlich M, Gandy S, Katsel P, Haroutunian V, Schadt E, Zhang B, 2018. The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease. Sci. Data 5, 180185. 10.1038/sdata.2018.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Ding SL, Li Y, Royall J, Feng D, Lesnar P, Graddis N, Naeemi M, Facer B, Ho A, Dolbeare T, Blanchard B, Dee N, Wakeman W, Hirokawa KE, Szafer A, Sunkin SM, Oh SW, Bernard A, Phillips JW, Hawrylycz M, Koch C, Zeng H, Harris JA, Ng L, 2020. The allen mouse brain common coordinate framework: a 3D reference atlas. Cell 181 (4), 936–953 e920. 10.1016/j.cell.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Shao W, Huang Z, Tang H, Zhang J, Ding Z, Huang K, 2021. MOGONET integrates multi-omics data using graph convolutional networks allowing patient classification and biomarker identification. Nat. Commun 12 (1), 3445. 10.1038/s41467-021-23774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pandey AK, Mulligan MK, Williams EG, Mozhui K, Li Z, Jovaisaite V, Quarles LD, Xiao Z, Huang J, Capra JA, Chen Z, Taylor WL, Bastarache L, Niu X, Pollard KS, Ciobanu DC, Reznik AO, Tishkov AV, Zhulin IB, Peng J, Nelson SF, Denny JC, Auwerx J, Lu L, Williams RW, 2016b. Joint mouse–human phenome-wide association to test gene function and disease risk. Nat. Commun 7 (1), 10464. 10.1038/ncomms10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Thibeau-Sutre E, Diaz-Melo M, Samper-Gonzalez J, Routier A, Bottani S, Dormont D, Durrleman S, Burgos N, Colliot O, Alzheimer’s Disease Neuroimaging, I, Australian Imaging, B, Lifestyle Flagship Study of, A, 2020. Convolutional neural networks for classification of Alzheimer’s disease: overview and reproducible evaluation. Med. Image Anal 63, 101694 10.1016/j.media.2020.101694. [DOI] [PubMed] [Google Scholar]
- Weston AD, Hood L, 2004. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J. Proteome Res 3 (2), 179–196. 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- Whitesell JD, Buckley AR, Knox JE, Kuan L, Graddis N, Pelos A, Mukora A, Wakeman W, Bohn P, Ho A, Hirokawa KE, Harris JA, 2019. Whole brain imaging reveals distinct spatial patterns of amyloid beta deposition in three mouse models of Alzheimer’s disease. J. Comp. Neurol 527 (13), 2122–2145. 10.1002/cne.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Jack CR, Josephs KA, 2012. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol. 11 (10), 868–877. 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Graff-Radford J, Tosakulwong N, Weigand SD, Machulda M, Senjem ML, Schwarz CG, Spychalla AJ, Jones DT, Drubach DA, Knopman DS, Boeve BF, Ertekin-Taner N, Petersen RC, Lowe VJ, Jack CR Jr., Josephs KA, 2018. [(18) F]AV-1451 clustering of entorhinal and cortical uptake in Alzheimer’s disease. Ann. Neurol 83 (2), 248–257. 10.1002/ana.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Holland D, Rongve A, Borte S, Winsvold BS, Drange OK, Martinsen AE, Skogholt AH, Willer C, Brathen G, Bosnes I, Nielsen JB, Fritsche LG, Thomas LF, Pedersen LM, Gabrielsen ME, Johnsen MB, Meisingset TW, Zhou W, Proitsi P, Hodges A, Dobson R, Velayudhan L, Me Research T, Sealock JM, Davis LK, Pedersen NL, Reynolds CA, Karlsson IK, Magnusson S, Stefansson H, Thordardottir S, Jonsson PV, Snaedal J, Zettergren A, Skoog I, Kern S, Waern M, Zetterberg H, Blennow K, Stordal E, Hveem K, Zwart JA, Athanasiu L, Selnes P, Saltvedt I, Sando SB, Ulstein I, Djurovic S, Fladby T, Aarsland D, Selbaek G, Ripke S, Stefansson K, Andreassen OA, Posthuma D, 2021. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet 53 (9), 1276–1282. 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP, 2012. The exposome: from concept to utility. Int. J. Epidemiol 41 (1), 24–32. 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, Arthur BJ, Bruns C, Rokicki K, Schauder D, Olbris DJ, Murphy SD, Ackerman DG, Arshadi C, Baldwin P, Blake R, Elsayed A, Hasan M, Ramirez D, Dos Santos B, Weldon M, Zafar A, Dudman JT, Gerfen CR, Hantman AW, Korff W, Sternson SM, Spruston N, Svoboda K, Chandrashekar J, 2019. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 179 (1), 268–281 e213. 10.1016/j.cell.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Klein GP, Silverstone FA, Levy AP, 1992. Nutritional patterns and weight change in Alzheimer patients. Int. Psychogeriatr 4 (1), 103–118. 10.1017/s1041610292000930. [DOI] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brašíc JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ, 2010. In vivo imaging of amyloid deposition in Alzheimer’s disease using the novel radioligand [18F]AV-45 (Florbetapir F 18). J. Nucl. Med 51 (6), 913–920. 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worheide MA, Krumsiek J, Kastenmuller G, Arnold M, 2021. Multi-omics integration in biomedical research - a metabolomics-centric review. Anal. Chim. Acta 1141, 144–162. 10.1016/j.aca.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wu C, Pan W, Alzheimer’s Disease Neuroimaging, I, 2017. Imaging-wide association study: Integrating imaging endophenotypes in GWAS. NeuroImage 159, 159–169. 10.1016/j.neuroimage.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Onos KD, Choi K, Keezer KJ, Skelly DA, Carter GW, Howell GR, 2021. Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep. 34 (6), 108739 10.1016/j.celrep.2021.108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Song W, 2013. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 250, 140–150. 10.1016/j.neuroscience.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T, 2008. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol. Psychiatry 64 (10), 835–841. 10.1016/j.biopsych.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Yates SC, Groeneboom NE, Coello C, Lichtenthaler SF, Kuhn PH, Demuth HU, Hartlage-Rubsamen M, Rossner S, Leergaard T, Kreshuk A, Puchades MA, Bjaalie JG, 2019. QUINT: workflow for quantification and spatial analysis of features in histological images from rodent brain. Front. Neuroinform 13, 75. 10.3389/fninf.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PNE, Estarellas M, Coomans E, Srikrishna M, Beaumont H, Maass A, Venkataraman AV, Lissaman R, Jiménez D, Betts MJ, McGlinchey E, Berron D, O’Connor A, Fox NC, Pereira JB, Jagust W, Carter SF, Paterson RW, Schöll M, 2020. Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res. Ther 12 (1), 49. 10.1186/s13195-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufov M, Weyandt LL, Piryatinsky I, 2017. Alzheimer’s disease and diet: a systematic review. Int. J. Neurosci 127 (2), 161–175. 10.3109/00207454.2016.1155572. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang Y, Zhou L, Yuan H, Shen D, Alzheimer’s Disease Neuroimaging, I, 2011. Multimodal classification of Alzheimer’s disease and mild cognitive impairment. NeuroImage 55 (3), 856–867. 10.1016/j.neuroimage.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mormino EC, Sun N, Sperling RA, Sabuncu MR, Yeo BT, Alzheimer’s Disease Neuroimaging, I, 2016. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 113 (42), E6535–E6544. 10.1073/pnas.1611073113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Hu Y, Zang T, Wang Y, 2019. Integrate GWAS, eQTL, and mQTL data to identify Alzheimer’s disease-related genes. Front. Genet 10 10.3389/fgene.2019.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fang J, Bekris LM, Kim YH, Pieper AA, Leverenz JB, Cummings J, Cheng F, 2021. AlzGPS: a genome-wide positioning systems platform to catalyze multi-omics for Alzheimer’s drug discovery. Alzheimers Res. Ther 13 (1), 24. 10.1186/s13195-020-00760-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi J, Guillaume JF, Neunlist M, Chaffron S, 2021. MiBiOmics: an interactive web application for multi-omics data exploration and integration. BMC Bioinform. 22 (1), 6. 10.1186/s12859-020-03921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


