Abstract
Cardiovascular disease and cancer are the two leading causes of morbidity and mortality in the world. The emerging field of cardio-oncology has revealed that these seemingly disparate disease processes are intertwined, owing to the cardiovascular sequelae of anticancer therapies, shared risk factors that predispose individuals to both cardiovascular disease and cancer, as well the possible potentiation of cancer growth by cardiac dysfunction. As a result, interest has increased in understanding the fundamental biological mechanisms that are central to the relationship between cardiovascular disease and cancer. Metabolism, appropriate regulation of energy, energy substrate utilization, and macromolecular synthesis and breakdown are fundamental processes for cellular and organismal survival. In this Review, we explore the emerging data identifying metabolic dysregulation as an important theme in cardio-oncology. We discuss the growing recognition of metabolic reprogramming in cardiovascular disease and cancer and view the novel area of cardio-oncology through the lens of metabolism.
The new field of cardio-oncology has blossomed because of the rapid growth in novel therapies for cancer. These treatments have revolutionized the overall prognosis and survival of patients with cancer, but cardiovascular and metabolic toxicities can occur1. Moreover, the intersection between cancer and cardiovascular disease (CVD) extends beyond toxicology2. Indeed, emerging data suggest that CVDs might potentiate cancer (a concept referred to as reverse cardio-oncology)3. One emerging aspect of this interaction is the metabolic milieu and metabolic switches that occur in both CVD and cancer. Tumours develop metabolic phenotypes that are distinct from those of adjacent, non-malignant tissue and, while providing cell-autonomous benefits for tumour growth, can also have cardiovascular and metabolic sequelae4–6. In addition, shared risk factors such as diabetes mellitus and obesity can predispose individuals to both CVD and cancer7,8. This concept has important public health implications and is especially relevant to a growing number of survivors of cancer, who are at high risk of developing CVD9–13. Indeed, several lines of evidence indicate that metabolism is a central mechanism in both CVD and cancer. Cross-disciplinary and cooperative research studies between cardiology and oncology are needed to translate findings from animal models to clinical applications, to improve patient care, and to use patient-derived samples for risk stratification and mechanistic studies.
In this Review, we highlight emerging themes in the field of cardio-oncology. We specifically look at these issues from the standpoint of metabolism, focusing on conceptual advances and the latest discoveries in the development of CVDs during tumour progression, with particular attention to how evolving metabolic and immunometabolic dependencies provide opportunities for therapeutic intervention to improve the care of patients with cancer and survivors of cancer14–16.
Metabolism in cardiac and cancer cells
Metabolism is a defining feature of every living cell, providing energy, biosynthetic intermediates and defence mechanisms against reactive by-products of oxidative metabolism. The variations in metabolic profile between tissues or cells are defined by the metabolic pathways that are being used and the flux rates through these pathways. Cardiomyocytes and cancer cells share a unique capacity to maintain crucial cellular functions during periods of stress17. One governing factor is the demand for ATP and macromolecule synthesis in the form of proteins, lipids or complex sugars. The heart achieves a continuous supply of ATP for contractile activities through tight coupling between substrate uptake and oxidation, while maintaining the synthesis of structural proteins and lipids. The primary catabolic demands of cardiomyocytes are met predominantly by using fatty acids, which are preferred over carbohydrates (such as glucose) and amino acids under normal physiological conditions18–20.
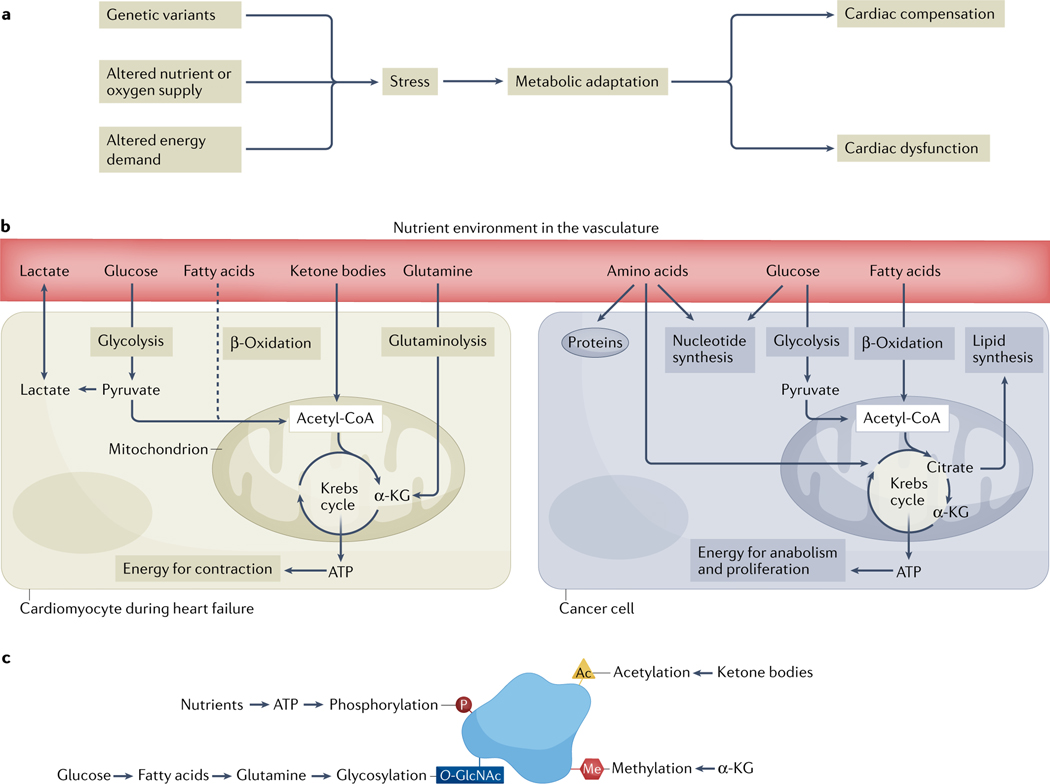
Various forms of stress, including increased physical activity, alterations in the blood composition of nutrients or reduced supply of oxygen, can challenge cardiac metabolism and cause a mismatch between ATP demand and oxidative processes (FIG. 1a). For example, in the failing heart, cardiac metabolism shifts from oxidative phosphorylation to glycolytic ATP provision, which allows cardiac contractile function to be maintained and creates a metabolic profile that is similar to that of tumour cells4,21. During heart failure progression, the utilization of amino acids and ketone bodies increases relative to that of fatty acids and carbohydrates22,23 (FIG. 1b). The degradation of glutamine and ketone bodies leads to the incorporation of carbons into the Krebs cycle via acetyl-CoA.
Fig. 1 |. The central role of metabolic remodelling in cardiovascular disease and cancer.
a | An overview of the metabolic consequences of stressors to the heart, which initially might be compensated for, but in the long term lead to dysfunction of the heart. b | In adult cardiomyocytes (left), the main source of fuel under normal physiological conditions is fatty acids. Stress initiates a shift in nutrient utilization away from fatty acid oxidation (dashed line) and towards glucose, ketone bodies or amino acids (such as glutamine) as sources of energy. These dynamic changes ensure continued ATP provision and maintenance of cardiac contractile function. In cancer cells (right), metabolic reprogramming supports successful adaptation to acquired mutations during tumorigenesis. Both catabolic and anabolic processes are maintained to ensure ATP provision and macromolecule synthesis during tumour growth. c | Post-translational modifications of proteins are linked to energy substrate metabolism and have a key role in the regulation of signalling, gene expression, protein stability and interactions, and enzyme kinetics. Ac, acetyl; α-KG, α-ketoglutarate; Me, methyl; O-GlcNAc, O-linked β-N-acetylglucosamine; P, phosphate.
Switches in metabolism not only have consequences for energy expenditure, but specific substrates can also function as signalling factors. For example, ketone bodies can act as a metabolic fuel, can function as an external signal by binding to cell-surface proteins and can promote epigenetic modifications by increasing the post-translational modification of histones via lysine acetylation24,25 (FIG. 1c). Likewise, glucose is a primary source of energy, but in excess amounts can be shuttled to the hexosamine pathway, resulting in O-linked β-N-acetylglucosamine (O-GlcNAc) modification of cytoprotective peptides26,27. The contribution of glutamine or other amino acids to cardiac energy substrate metabolism under normal physiological conditions is negligible but increases substantially during pathological remodelling and in the failing heart28,29. Studies indicate that glutamine-derived carbons are used to maintain ATP provision, whereas glutamine-derived nitrogen is donated for macromolecule synthesis30. Branched-chain amino acid catabolism is disrupted during heart failure, in which the oxidation of branched-chain amino acids (valine, leucine and isoleucine) is substantially downregulated, causing the accumulation of catabolic by-products31,32. The notion that energy substrates are only a fuel is, therefore, incorrect because it neglects the pleiotropic roles of metabolic factors in the internal milieu.
In the heart, metabolic changes are directly linked to organ dysfunction or preservation, whereas in tumours, metabolic remodelling supports the acquisition and maintenance of malignancy. Cancer cells alter metabolic pathways by balancing catabolic and anabolic requirements to meet cellular homeostatic, bioenergetic and biosynthetic requirements (FIG. 1b). These findings have led to the perception that cancer cells have a defined metabolic profile, but in vivo studies of cancer metabolism have challenged this view33,34. The Warburg effect, characterized by a preference for glycolysis and increased secretion of lactate, even in the presence of oxygen, is an example of oncogenic remodelling in many proliferating cancer cells35,36. Although the initial interpretation was that oxidative metabolism is deficient in tumours, subsequent studies demonstrated that cancer cells retain their capacity for mitochondrial respiration with increased glycolysis, suggesting that the regulation of glycolysis is impaired37.
Studies indicate that the metabolic phenotype of tumours is heterogeneous, dynamic and flexible, and this view is supported by insights from advanced technologies, including mass spectrometry-based metabolomics and proteomics, functional genomics and computational metabolic flux analyses in mouse models of cancer and patients with cancer33,38–41. Furthermore, metabolic phenotypes in tumours evolve as the tumour progresses from premalignant lesions to locally invasive and eventually metastatic cancer. Oncogene-driven expression of nutrient transporters42,43, autophagic degradation of proteins and organelles44 and environmental factors through the tumour microenvironment influence metabolic differences between tumours, and can also give rise to regional heterogeneity within a single tumour34,38–41.
Tumorigenic variants in KRAS, TP53 and MYC (encoding GTPase KRas, cellular tumour antigen p53 and MYC proto-oncogene protein, respectively) drive metabolic remodelling in cancer cells by accommodating the increased demand for nutrients to support cell proliferation42,43,45,46. Variants in KRAS can increase the expression of amino acid transporter SLC7A5 and autophagic flux, thereby supporting the higher demand for protein synthesis in proliferating cancer cells42. Likewise, p53 and MYC control various metabolic pathways and transporter activities for nutrients. p53 is a central component of cellular stress response pathways, and various forms of stress (including nutrient deprivation) can lead to p53 activation via the AKT–mTOR signalling pathway and AMP-activated protein kinase47,48. Nucleocytoplasmic malate dehydrogenase 1 has been shown to bind to and activate p53 in response to glucose deprivation, leading to increased oxidative metabolism49. Oncoproteins of the MYC family are crucial drivers of malignancy and are deregul ated in up to 70% of human cancers through several mechan isms, including genetic variants, super-enhancer activation, aberrant upstream signalling and altered protein turnover45,46,50. Studies have demonstrated that increased MYC expression drives metabolic regulation of macromolecule synthesis and building blocks (such as lipids, nucleic acids and proteins) to sustain increased cancer cell proliferation51. KRAS, p53 and MYC directly affect the transcription of glycolytic enzymes52,53. In particular, MYC modulates the expression of glucose transporter SLC2A1, lactate exchange via monocarboxylate transporter 1 (MCT1) and MCT2, and several glycolytic enzymes21,54. Together, these studies indicate the tight link between metabolism and gene expression regulation to control the adaptation of cancer cells to stress.
Shared risk factors for CVD and cancer
CVD and cancer share several risk factors, including diabetes, dyslipidaemia, cachexia and an impaired immune response14,15,55. In patients with obesity and diabetes, the plasma availability of glucose is increased, the abundance and composition of plasma lipids are altered, insulin regulation is disrupted and the levels of inflammatory cytokines are upregulated56–58. Likewise, cancer is a systemic disease that affects the cardiovascular system through several factors, including the release of small molecules, modulation of immune cell activity and metastatic lesions.
CVD and cancer frequently coincide in the same patient and often complicate each other. To date, much of the focus in cardio-oncology has been on the cardiovascular complications developed during cancer progression and as a result of cancer treatment59,60. However, the reverse can also be true, and patients with CVD have been shown to be at increased risk of developing cancer (reverse cardio-oncology3), as reviewed previously61,62.
Multiple pathways and mechanisms have been proposed for the comorbidity of CVD and cancer2,3,62–66. First, CVD and cancer share environmental risk factors, including obesity, smoking and a sedentary lifestyle7,67,68. Furthermore, traditional cardiovascular risk factors, such as dyslipidaemia and hypertension, can also be associated with the development of cancer — commonly used 10-year risk scores for atherosclerotic CVD are also predictive of incident cancer8. This finding emphasizes that the risk factors for CVD and cancer overlap.
Second, shared genetic variants might explain the connection between CVD and cancer. Specific inherited genetic variants (for example, in genes encoding components of the WNT signalling pathway, the DYRK protein kinase family and the methionine pathway) have been associated with both incident cancer and incident CVD, such as coronary artery disease and heart failure69,70. Moreover, clonal haematopoiesis of indeterminate potential (CHIP), which is caused by certain somatic mutations in haematopoietic stem cells, has been identified as a shared risk factor for the onset and development of both CVD and cancer71–73. CHIP increases the risk of blood cancers, cardiometabolic diseases and microvascular dysfunction74,75. Remarkably, the risk of cardiovascular events is doubled in patients with CHIP76. Approximately 80% of the mutations occur in genes encoding epigenetic regulators, such as DNMT3A and TET2 (REFS77–79). Somatic mutations in DNMT3A and TET2 contribute to the development of atherosclerosis through increased endothelial inflammation driven by molecular interactions between circulating clonal monocytes and macrophages and the endothelium80. TET2-deficient macrophages have increased IL-1β secretion, which modulates endothelial cell adhesion and vascular permeability81. Heritable and acquired risk factors, including age, unhealthy lifestyle behaviours (for example, smoking and obesity), inflammatory conditions and exposure to anticancer therapies, are associated with an increased prevalence of CHIP82. For example, the incidence of CHIP among patients treated with stem cell transplantation for lymphoma was nearly 30%71. Although CHIP greatly increases the risk of haematological malignancies, the main cause of death in individuals with CHIP is atherosclerotic CVD6,74,76. Whether CHIP is a causal risk factor for CVD or simply reflects the accumulation of somatic mutations during biological ageing has been debated. However, it has been established that the presence of CHIP alters the function of immune cells, such as macrophages, which at least partly explains the increased propensity to develop coronary artery disease and its complications, as well as adverse myocardial remodelling76,77,79. Furthermore, DNMT3A mutations increase platelet production, which can be accompanied by increased platelet functionality, leading to a higher risk of cardiovascular events83.
Third, inflammation is a central driver in both CVD and cancer. The CANTOS trial84 evaluated the use of canakinumab, a human monoclonal antibody to IL-1β, in >10,000 patients with previous myocardial infarction and a blood level of high-sensitivity C-reactive protein of ≥2 mg/dl. Compared with placebo, canakinumab treatment was associated with a significantly lower rate of recurrent cardiovascular events, independent of LDL-cholesterol levels84. Strikingly, canakinumab treatment also reduced the incidence of lung cancer, although this outcome was a secondary end point of the trial85. Prospective studies evaluating the efficacy of canakinumab are ongoing, but the results from the CANTOS trial suggest that targeting inflammation can both reduce the risk of CVD and limit tumour growth. The use of other compounds that interfere with IL-1 signalling, such as anakinra (a recombinant and slightly modified version of the human protein IL-1 receptor antagonist), has also been associated with reductions in both cardiovascular events86 and cancer events87. In addition, the use of generic anti-inflammatory drugs, such as colchicine, is effective in reducing CVD events88,89, although the efficacy in patients with cancer is uncertain. Clearly, inflammation itself is heterogeneous, but the emerging data that inflammation has a central role in both CVD and cancer calls for a greater understanding of the underlying mechanisms.
Cancer metabolism and cardiovascular remodelling
Metabolic dysregulation of cancer cells can extend beyond the tumour microenvironment and lead to both systemic and cardiac-specific consequences (FIG. 2). The best evidence for tumour-intrinsic factors causing cardiov ascular dysregulation comes from variants in genes encoding metabolic enzymes that can lead to cancer but which can also have systemic repercussions (FIG. 3). For example, somatic mutations in IDH1 and IDH2 (encoding cytosolic isocitrate dehydrogenase [NADP] (IDH1) and mitochondrial isocitrate dehydrogenase [NADP](IDH2), respectively) have been identified in gliomas (82%), acute myeloid leukaemia (15%), colorectal cancer (10%) and prostate cancer (1–3%)90,91. Variants in IDH1 and IDH2 lead to excessive accumulation of the oncometabolite d-2-hydroxyglutarate (D2-HG) in cancer cells and subsequent release into the bloodstream. D2-HG promotes epigenetic modifications via inhibition of α-ketoglutarate-dependent dioxygenases, which in turn provides a benefit to tumours for growth and proliferation92–94 (FIG. 4). In addition, multiple preclinical studies have shown that D2-HG affects the cellular functions of non-malignant cells. Increased production and release of D2-HG by cancer cells with IDH1 or IDH2 variants impairs oxidative metabolism via inhib ition of the α-ketoglutarate dehydrogenase and inhibits ATP provision and cardiac contractile function4,95. Additionally, D2-HG contributes to an immunosuppressive milieu by impairing the immune cell response via inhibition of T cell activation and proliferation96,97. Together, these systemic effects might explain observations that show an association between the presence of IDH1 or IDH2 variants in patients with leukaemia and reduced left ventricular function, especially after chemotherapy with anthracyclines, which has cardiotoxic effects98. The effects of D2-HG are enantiomer-specific and can be reversible, offering the potential for compounds that block variants of IDH1 or IDH2 to have antitumour activity.
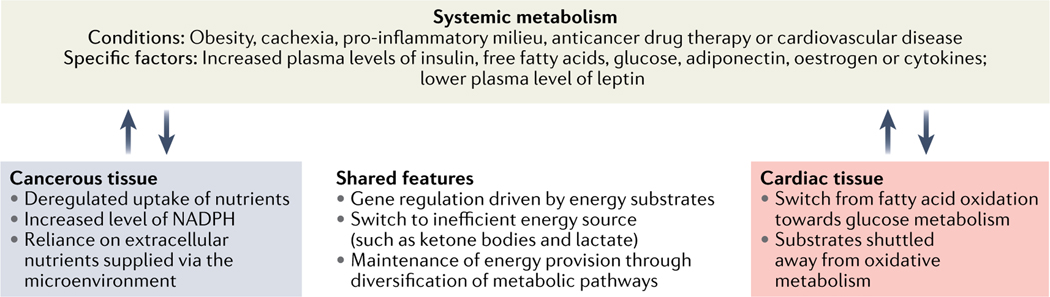
Fig. 2 |. Metabolism bridges cancer and cardiovascular disease.
Several metabolic stressors and perturbations, including obesity and cachexia, prompt the production and release of metabolic and inflammatory signal peptides and molecules. These systemic effects are accompanied by distinct differences as well as shared features in cardiac tissue and in cancer cells.
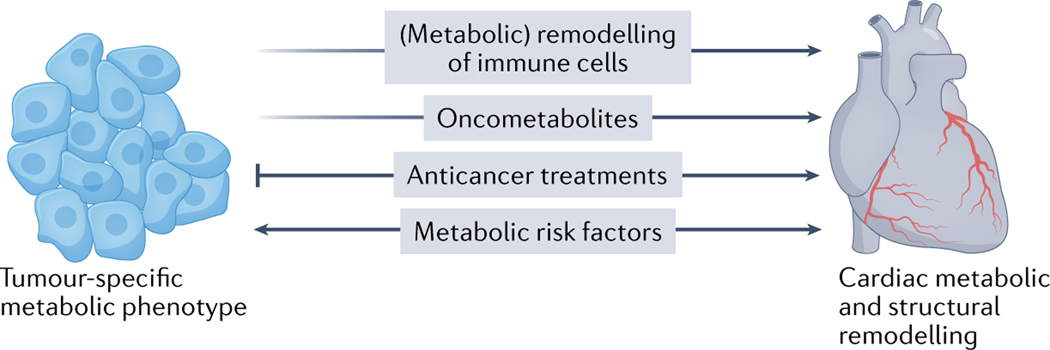
Fig. 3 |. Putative mechanisms of cardio-onco-metabolic remodelling.
The presence of cancer and/or the use of anticancer therapies can provoke changes in the organism, such as remodelling of immune cells, that affect the heart. Furthermore, specific oncometa bolites, such d-2-hydroxyglutarate and succinate, can affect the heart tissue directly. Metabolic risk factors can cause cardiovascular disease as well as exacerbate tumour proliferation and cancer progression.
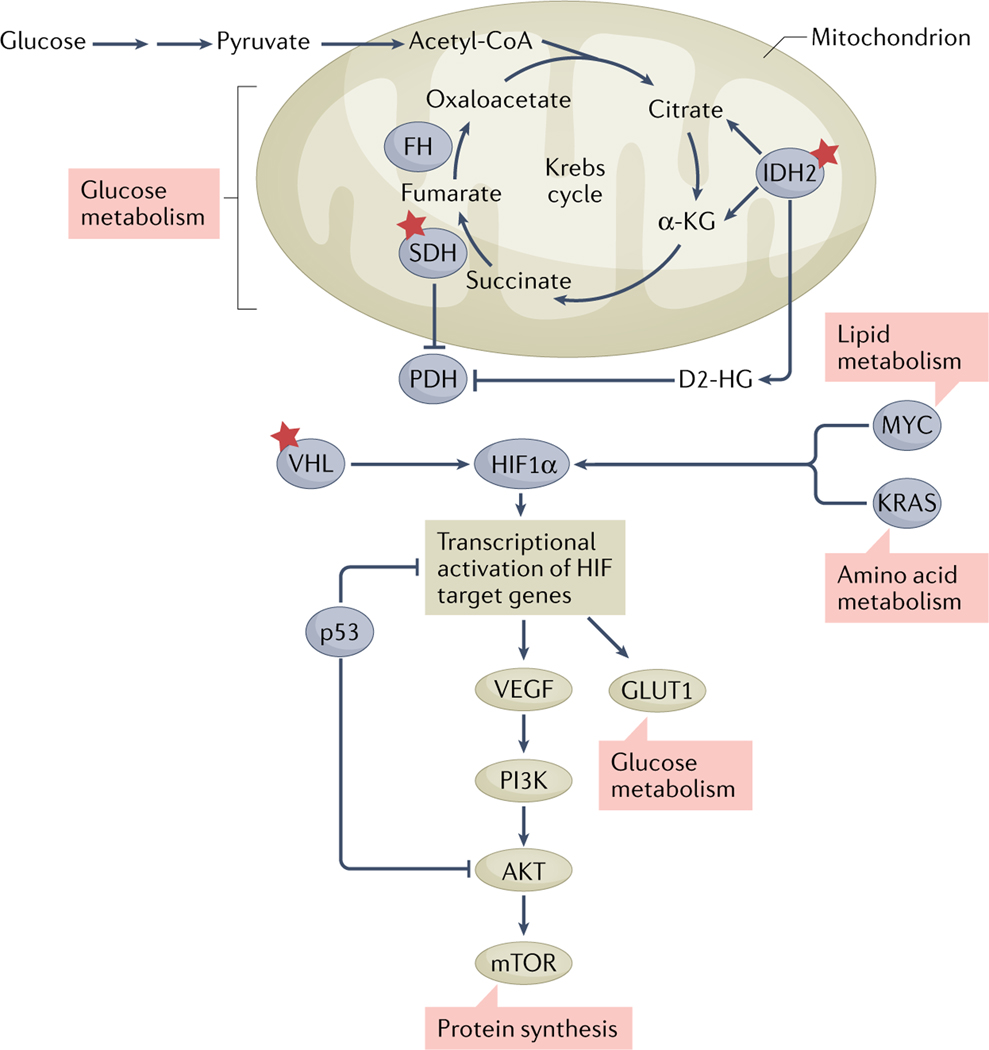
Fig. 4 |. Accumulation of somatic mutations changes the metabolic profile of tumours and influences the cardiovascular system.
Variants (indicated by red stars) in IDH1 or IDH2, encoding cytosolic isocitrate dehydrogenase [NADP] (IDH1) and mitochondrial isocitrate dehydrogenase [NADP] (IDH2), cause increased production and release of the oncometabolite d-2-hydroxyglutarate (D2-HG). D2-HG promotes epigenetic modifications and tumorigenesis. Variants in VHL, encoding von Hippel–Lindau disease tumour suppressor (VHL), are associated with vascular tumours by interference with the hypoxia-inducible factor 1α (HIF1α)–vascular endothelial growth factor (VEGF) pathway. Likewise, variants in succinate dehydrogenase (SDH)-encoding genes increase malignant remodelling and affect transcriptional regulation. α-KG, α-ketoglutarate; AKT, RACα serine/threonine-protein kinase; FH, mitochondrial fumarate hydratase; GLUT1, glucose transporter type 1; KRAS, GTPase KRas; mTOR, mechanistic target of rapamycin; MYC, MYC proto-oncogene protein; p53, cellular tumour antigen p53; PDH, pyruvate dehydrogenase; PI3K, phosphoinositide 3-kinase.
The overall effect of cancer on the cardiovascular system depends on the size of the tumour, its vascularization, the shielding of the tumour from the invaded organ(s) (for example, by the presence of a capsule) and several other factors. This concept has been most studied for the oncometabolite D2-HG. The production and release of D2-HG has been directly linked to the development of cardiomyopathy and neurological disorders4,5. 2-Hydroxyglutaric acidurias are a heterogeneous group of genetic diseases that are characterized by the accumulation of D2-HG or l-2-hydroxyglutarate (L2-HG) in bodily fluids and which are caused by variants in D2HGDH (encoding mitochondrial D2-HG dehydrogenase), L2HGDH (encoding mitochondrial L2-HG dehydrogenase), IDH2 or SLC25A1 (encoding the mitochondrial tricarboxylate transport protein). The mitochondrial D2-HG and L2-HG dehydrogenases catalyse the conversion of D2-HG and L2-HG, respectively, to α-ketoglutarate. Loss-of-function variants in D2HGDH or L2HGDH cause an accumulation of D2-HG or L2-HG, respectively, and impairment of endogenous enzymatic systems99. Children with these variants have a wide range of neurological disorders as well as dilated or hypertrophic cardiomyopathy100. Interestingly, adult patients with 2-hydroxyglutaric aciduria syndrome often harbour heterozygous germline variants in IDH2 in addition to variants in D2HGDH or L2HGDH, resulting in even higher levels of D2-HG and L2-HG and a substantially increased risk of cardiomyopathy101,102. In adult mice, global induction of variant Idh2 expression (and the subsequent increase in plasma D2-HG and L2-HG levels) resulted in dilated cardiomyopathy, muscular dystrophy and white matter abnormalities throughout the central nervous system5. Hearts from these mice accumulated glycogen and had smaller and fewer mitochondria than hearts from healthy control mice5. Remarkably, implantation of tumour xenografts harbouring an IDH2 variant also resulted in cardiac abnormalities5, suggesting that D2-HG and L2-HG can act in a paracrine fashion to cause cardiotoxicity.
In individuals with somatic mutations in IDH1 or IDH2, paracrine or endocrine effects can be the cause of cardiac remodelling, but in other situations, inherited genetic variants can directly cause metabolic dysregulation. Biallelic loss-of-function variants in VHL (encoding von Hippel–Lindau disease tumour suppressor), which are typical in renal cell carcinoma and haemangioblastoma, are an example of how metabolic reprogramming facilitates the development of cancer and can lead to cardiac sequelae103,104 (FIG. 4). von Hippel–Lindau syndrome, characterized by germline variants in VHL, predisposes patients to a multitude of vascular tumours, including retinal angiomas and cerebellar haemangioblastomas103. Furthermore, patients with specific homozygous variants in VHL can have polycythaemia and cardiopulmonary abnormalities, including increased basal ventilation, pulmonary vascular tone and heart rate responses at baseline, and these abnormalities are accentuated by hypoxia105,106. During exercise, these patients have early and marked phosphocreatine depletion and acidosis in skeletal muscle, greater accumulation of lactate in the blood and reduced maximum exercise capacity107. Transgenic mice with the same Vhl variants have increased glycolysis and a decreased phosphocreatine to ATP ratio in the heart, consistent with impaired oxidative metabolism108. A case study described a patient with a point mutation in VHL that was associated with reduced growth rate, persistent hypoglycaemia and limited exercise capacity, with gene expression changes that reprogrammed carbohydrate and lipid metabolism, impaired mitochondrial respiratory function in skeletal muscle and uncoupled oxygen consumption from ATP production109. Finally, cardiac-specific deletion of Vhl in mice can lead to progressive heart failure and premature death, with a subset of mice developing malignant cardiac tumours with features of rhabdomyosarcoma and the capacity to metastasize110.
The protein product of VHL functions as an E3 ubiquitin ligase of hypoxia-inducible factor 1α (HIF1α), which promotes proteasome-mediated degradation of HIF1α (and of the related protein, HIF2α) during normoxia. In hypoxia, HIF1α and HIF2α are stable and heterodimerize with HIF1β (also known as ARNT protein) to function as a master transcription factor for the induction of hundreds of genes that are crucial for the cellular and systemic response to hypoxia. Tumours with VHL variants have aberrant activation of HIF2α and, therefore, show many of the hallmarks of hypoxia111. HIF2α (the HIF isoform specifically implicated in renal cell carcinoma) activates the transcription of genes encoding angiogenic growth factors (such as members of the vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) families) as well as genes encoding metabolism-related protein (such as the glucose transporter type 1), thereby changing the metabolic phenotype of affected tumours112,113. Belzutifan, a small-molecule inhibitor of HIF2α, has been approved for the treatment of patients with von Hippel–Lindau syndrome114. In a mouse xenograft model of renal cell carcinoma, inhibitors of HIF2α blocked the angiogenic and metabolic targets of HIF2α, demonstrating on-target antitumour activity112,115. Interestingly, in mouse models with the specific variants in Vhl that result in polycythaemia, treatment with a HIF2α inhibitor reversed the cardiopulmonary phenotypes associated with the genetic variant116. The spectrum of cardiopulmonary abnormalities that are associated with VHL variants is a demonstration of the genotype–phenotype correlations that occur between cancer-associated variants and cardiovascular metabolism.
The systemic and cardiac effects of the presence in tumours of other inherited or somatic variants in genes encoding metabolic enzymes are less clear. Biallelic (germline or somatic) loss-of-function variants in genes encoding subunits of succinate dehydrogenase (SDH) can cause rare conditions that comprise 2% of mitochondrial respiratory chain disorders117 (FIG. 4). SDH forms complex II of the mitochondrial electron transport chain and couples the oxidation of succinate to fumarate in the Krebs cycle to the transfer of electrons to the terminal acceptor ubiquinone in the electron transport chain. The four subunits of the SDH complex are encoded by SDHA, SDHB, SDHC and SDHD in the nucleus. Genetic variants in one or more subunits predispose individuals to a variety of tumours, including phaeochromocytoma, paraganglioma, gastrointestinal stromal tumours, haemangioblastoma and papillary renal cell carcinoma117–122. Germline variants in SDH-encoding genes are associated with the complete loss of enzymatic function and mitochondrial accumulation of succinate123. The cardiac phenotypes of patients who have tumours with variants in SDH-encoding genes are yet to be defined in a large population, but case reports suggest that these variants are associated with severe myopathy, most notably dilated cardiomyopathy with impaired left ventricular function124–128.
Like D2-HG, succinate is considered to be an oncometabolite, and drives genome-wide hypermethylation and transcription factor activation via inhibition of α-ketoglutarate-dependent dioxygenases and HIF1α prolyl hydroxylases122,123. However, whether succinate drives tumorigenesis and acts as a metabolic signal during malignancy remains to be determined. Increased plasma succinate levels are associated with CVDs and increased inflammation, as well as ischaemia–reperfusion injury129,130. Preclinical studies suggest that succinate release from cancer cells activates an immune response and cellular signalling via succinate receptor 1 (REFS131,132). Despite these promising preclinical studies, further research is needed to explain the cardiometabolic phenotype associated with variants in SDH-encoding genes.
A more general effect of cancer on the cardiovascular system is cancer-associated cachexia, a debilitating condition characterized by skeletal muscle wasting and loss of adipose tissue, which substantially contributes to morbidity and mortality133,134. Many factors contribute to cancer-induced muscle wasting, including altered protein and energy metabolism and chronic inflammation134–136. Pro-inflammatory cytokines, including tumour necrosis factor, IL-1β and IL-6, which are produced either by cancer cells or by immune cells in response to the tumour, interfere with appetite signals in the anterior hypothalamus and increase the metabolic rate137. Increased net protein breakdown and increased oxidation of branched-chain amino acids are characteristic features of solid tumours, and result in decreased plasma amino acid concentrations42,138. Accordingly, monitoring of plasma amino acid levels (for example, glutamine) has emerged as a pretreatment risk stratification tool138, and controlling amino acid availability is a promising therapeutic intervention139,140.
Metabolic effects of anticancer therapies
Anticancer therapies can induce CVD via several mechanisms, including direct cardiotoxicity, effects on the vasculature, and perturbations to cardiovascular and immune homeostasis141–144. In addition, a subset of anticancer therapies can have substantial metabolic effects, which can either manifest systemically or cause organ-specific perturbations (for example, in the heart). Traditional anticancer therapies, such as anthracyclines and radiation, have long been known to be associated with cardiovascular sequelae, including cardiomyopathy and cardiac ischaemia145. Because of the non-specific mechanisms of action of these therapies, the cardiotoxicities can involve direct cell death and also metabolic sequelae. For example, preclinical models suggest multiple mechanisms of anthracycline-mediated cardiotoxicity, with several studies indicating metabolic perturbations, such as impairment of mitochondrial biogenesis and iron metabolism and effects on transcription factors that regulate metabolism, including HIF60,146,147. Cardiotoxicity can also occur with therapy with antimetabolites, such as 5-fluorouracil, a synthetic analogue of uracil that inhibits thymidylate synthase, thereby limiting the availability of thymidine nucleotides for DNA synthesis. Although 5-fluorouracil is an effective anticancer treatment, cardiotoxicity can result from vascular spasms148. Androgen deprivation therapy (with the use of drugs such as leuprolide), which is a mainstay of treatment for prostate cancer, can cause systemic metabolic sequelae, including hyperglycaemia, hypertriglyceridaemia, increased adiposity and decreased lean body mass149.
An improved understanding of the specific pathways that are dysregulated in cancer has led to the development of more targeted therapies, but these therapies have been associated with more diverse metabolic dysregulation. For example, given that specific kinases become aberrantly activated in different types of malignancy, kinase inhibitors have emerged over the past two decades as important forms of anticancer treatment59. Kinase inhibitors can generally be divided into antibodies and small molecules. Small-molecule inhibitors bind to receptor kinases intracellularly, inhibiting the catalytic activity of tyrosine kinases by allosteric inhibition or by directly interfering with the binding of ATP to a structurally unique pocket. However, because the ATP-binding pocket can be similar on more than one kinase receptor, small-molecule inhibitors can target more than one kinase. For this reason, whereas biologic agents (such as antibodies) are often fairly specific, small molecules can be promiscuous and result in off-target inhibition of kinases other than the intended target150. Depending on the kinases affected, metabolic dysregulation can arise.
For example, small-molecule inhibitors targeting VEGF and PDGF receptors have been rapidly developed for the treatment of many forms of cancer, including kidney cancer143,151. These therapies are often associated with hypertension and are associated with mild cardiomyopathy152–154. VEGF is widely expressed in cardiac tissue, and inhibition of VEGF signalling can impair the growth, development and repair of cardiac tissue155,156. In addition, therapy with VEGF inhibitors can be associated with relative hypoglycaemia, although isolated cases of severe hypoglycaemia have been reported157. In experimental models, sunitinib (a small-molecule, multi-targeted receptor tyrosine kinase inhibitor) prevented and reversed diabetes in mice as a result of ‘on-target’ inhibition of both PDGF and VEGF signalling158,159. Interestingly, therapy with imatinib, which is primarily used to inhibit tyrosine protein kinase ABL1, which is activated in certain forms of leukaemia, is also associated with hypoglycaemia in experimental models and in patients160–163. Imatinib does not target VEGF receptors but was initially developed as a PDGF receptor inhibitor, which contributes to the changes in blood glucose levels, although the specific mechanisms are uncertain158,164. VEGF inhibitor therapy can lead to mild cardiomyopathy, which is often reversible after drug discontinuation152,165. Mechanistically, this cardiomyopathy arises owing to direct inhibition of VEGF and PDGF, resulting in microvascular dysfunction and stabilization of HIF and downstream targets166,167. In accordance with this mechanism, mice in which HIF is genetically stabilized also develop cardiomyopathy, which is reversible when the transgene is turned off168,169. Similarly, phosphoinositide 3-kinase (PI3K) inhibitors are associated with hyperglycaemia143. This adverse effect is expected, because PI3K is an important modulator of insulin signalling and lipid homeostasis162. Although the metabolic complications associated with VEGF or PI3K inhibitor therapies are often ‘on-target’ (that is, caused by the direct inhibition of the intended kinase target), the mechanisms of toxicity associated with other kinase inhibitors are less clear. Nilotinib, an ABL1 kinase inhibitor that is used in the treatment of some forms of leukaemia, is associated with hyperglycaemia and subsequent vascular disease162. The association between nilotinib and hyperglycaemia is presumably an off-target effect, because other ABL1 kinase inhibitors are not associated with hyperglycaemia, and imatinib is even associated with hypoglycaemia163.
In the past decade, intense efforts have been made to target the metabolism of the tumour or the tumour microenvironment in the treatment of cancer. Many of these efforts have been precision-based. For example, ivosidenib (an IDH1 inhibitor previously known as AG-120) and enasidenib (an IDH2 inhibitor previously known as AG-221) have been approved by the FDA for the treatment of patients with relapsed or refractory acute myeloid leukaemia and variants in IDH1 or IDH2, respectively170,171. These drugs are currently being tested in patients with other types of cancer with variants in IDH1 or IDH2, including glioma, cholangiocarcinoma and chondrosarcoma170–173. Any adverse sequelae of these therapies are currently uncertain because of their recent approval, although given that ivosidenib and enasidenib target only mutant IDH1 and IDH2, respectively, any effect on wild-type IDH enzymes in normal organs, including the heart, should be minimal. Interestingly, prolongation of the corrected QT interval (QTc) on the surface electrocardiogram, which increases the risk of ventricular arrhythmia, was a serious and unexpected adverse effect associated with ivosidenib in both preclinical and clinical testing174. Indeed, >25% of patients who were treated with ivosidenib had QTc prolongation, although only 8% required treatment interruption or dose reduction174.
Finally, immunotherapies have revolutionized anticancer treatment in the past decade175. Immunotherapies include a broad range of novel drugs, from antibodies and other biologic agents, including immune checkpoint inhibitors and bispecific T cell engagers, to cell-based therapies, such as chimeric antigen receptor T cell therapies. Immune checkpoint inhibitors can cause inflammatory toxicities, including myocarditis176; however, adverse effects can also include metabolic toxicities, including diabetes177. Cellular therapies can result in cytokine release syndrome, which can manifest with mild to life-threatening symptoms, including severe hypotension and vascular leak175,178–180. Although the mechanisms of these cardiovascular toxicities are not clear, metabolic perturbations resulting from cytokine release syndrome could contribute to systemic toxicities.
Targeting metabolism in CVD and cancer
Given that metabolic pathways are altered in both CVD and cancer, specific treatments that target metabolic features might be beneficial in both conditions. Genetically defined metabolic phenotypes contribute mechanistically to tumour transformation and are potential therapeutic targets. Targeting these metabolic signatures by inhibiting enzymatic functions or through dietary interventions holds the promise to alter tumour metabolite availability and influence cancer cell growth. Incorporating pharmacological and interventional treatments targeting metabolism might improve the efficacy of existing anticancer treatments and might also reduce the overall risks associated with cancer-associated CVD. Given the various possibilities, we restrict the following discussion to some specific examples.
First, variants in genes that encode metabolic enzymes are important drivers of tumour initiation and growth and can be targeted therapeutically. This paradigm has been successfully applied to tumours with variants in IDH-encoding genes. The clinical efficacy of inhibitors of mutant IDH1 and IDH2 has been demonstrated in patients with acute myeloid leukaemia151, and clinical trials in patients with advanced cholangiocarcinoma or chondrosarcoma have shown increased progression-free survival with the IDH1 inhibitor ivosidenib compared with placebo181,182 (FIG. 5). IDH1 inhibitors provide new options for patients with unresectable, metastatic and/or refractory cancer who have no other treatment options. Another benefit of IDH1 inhibitors is the significant reduction in plasma D2-HG concentrations, which might improve the D2-HG-mediated metabolic alterations observed in these patients181.
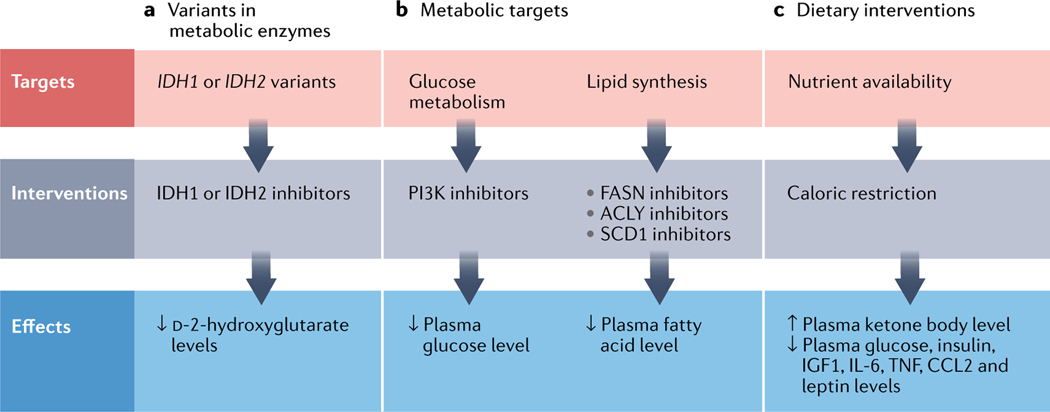
Fig. 5 |. Metabolic targets and interventions in cardiovascular disease and cancer.
a | Inhibitors targeting variant forms of cytosolic isocitrate dehydrogenase [NADP] (IDH1) or mitochondrial isocitrate dehydrogenase [NADP] (IDH2) are efficacious in patients with acute myeloid leukaemia. b | Inhibitors of the phosphoinositide 3-kinase (PI3K) pathway or of fatty acid synthase (FASN), ATP–citrate synthase (ACLY) or stearoyl-CoA desaturase 1 (SCD1) lower plasma levels of glucose or fatty acids and might have beneficial effects in patients with cardiovascular disease or cancer. c | Caloric restriction has beneficial effects in patients with cardiovascular disease or cancer via its pleiotropic effects on various metabolic and inflammatory components. CCL2, C-C motif chemokine 2; IGF1, insulin-like growth factor 1; TNF, tumour necrosis factor.
Second, obesity, diabetes and dyslipidaemia have established systemic manifestations, such as increased inflammation, which might explain their widespread and profound effects on the organism. Interventions that reverse the deleterious effects of these metabolic stressors might have beneficial effects on the risk of CVD and cancer. Dietary interventions have been proposed as another effective strategy to target cancer cells and reduce the risk of CVD (FIG. 5). Fasting is the most extreme approach to reset an organism’s metabolism and has been shown to have positive effects in cancer prevention and treatment in mice183. Just 1 day of fasting per week delays spontaneous tumorigenesis in p53-deficient mice184. Fasting is associated with decreases in plasma glucose, insulin and insulin-like growth factor 1 levels, which might partly explain the salutary effects of fasting185–187. Furthermore, fasting is followed by a period of abnormally high cellular proliferation, which is characterized by the activation of cellular repair pathways and is driven by the replenishment of growth factors during refeeding to reverse atrophic cellular remodelling183,184. No clinical data are currently available to advocate intermittent fasting in patients with cancer, but several trials are underway. Low glycaemic diets have been shown to reduce lipid metabolism and tumour growth188. Bariatric surgery, another drastic intervention to reduce obesity, has been shown to have long-term preventive effects on incident CVD and cancer189. Other examples of therapies that modify metabolism are drugs to reduce serum cholesterol levels, such as statins, which are extremely effective in preventing coronary and cerebrovascular events190. However, the effects of statins on the incidence of cancer are uncertain, but are likely to be neutral according to a meta-analysis190.
Combining metabolic inhibitors with anticancer therapies holds the promise to improve the efficacy and durability of existing treatments for patients with cancer, while also protecting the heart. Several approved metabolic therapies target lipid synthesis, but their clinical applications have been limited. Some tumours rely on glucose metabolism during the early stages of the disease. The use of PI3K inhibitors targeting glucose homeostasis and metabolism has been successful in the treatment of a subset of cancers, including breast cancers191,192 (FIG. 5). Inhibition of PI3K can lead to both decreased cancer cell proliferation and increased cellular death191. However, the use of PI3K inhibitors has been limited in some patients owing to fulminant hypoglycaemia and therapy resistance. Depending on the tumour profile, compensatory signalling mechanisms result in hyperinsulinaemia, causing increased tumour growth and treatment failure191,192.
Some types of tumour, such as breast cancer, rely on fatty acid synthesis in the advanced stages of the disease193. Targeting de novo fatty acid synthesis by inhibition of fatty acid synthase has been proposed as a promising therapeutic strategy in HER2+ breast cancer with brain metastasis193. Likewise, inhibitors of stearoyl-CoA desaturase and ATP–citrate synthase in tumours with increased lipid synthesis (such as colorectal cancers and pancreatic cancer) have shown promising results in preclinical studies194–196. The cardiovascular risk associated with these therapies is uncertain, but evidence from previous clinical trials suggests that these therapies are likely to be associated with increased risks of adverse cardiovascular events, perhaps owing to metabolic dysregulation149. Risk stratification of patients on the basis of existing metabolic risk factors, the tumour profile, cell-specific drug delivery and cardiac remodelling is necessary.
Conclusions
Metabolic adaptation in CVD and cancer is complex and dynamic. The causes of these metabolic changes are multifactorial, including intrinsic and extrinsic factors to both normal and diseased tissue. These complexities introduce challenges to elucidating how cancer cells affect other organs and potentially impair their function. Additional studies are necessary to predict metabolic signatures that can be therapeutically targeted and the potential systemic effects of these therapies. CVDs have emerged as a leading cause of death in survivors of cancer, prompting questions about how tumours alter cellular states beyond their own direct environment. The metabolism of cancer cells and cardiomyocytes seems to be different at first glance, but closer examination of the cellular processes shows that similar stress-response pathways exist in cardiomyocytes and certain types of tumour. Furthermore, tumours impose metabolic stress on the heart, which causes distinct metabolic phenotypes. Understanding the processes by which metabolic processes remodel is likely to provide new avenues of therapeutic interventions and to improve our understanding of cardiac adaptation. To move the field forwards, we need to harness new technologies in metabolic imaging and stable isotope tracer analysis, and to develop models that provide a bridge between preclinical discoveries and clinical translation.
Key points.
metabolic remodelling is a defining feature of both cardiovascular diseases and tumours.
metabolic dysregulation of cancer cells extends beyond the tumour microenvironment and can lead to both systemic and cardiac-specific consequences.
Cardiovascular disease and cancer share several risk factors, including diabetes mellitus, dyslipidaemia, cachexia and an impaired immune response.
Anticancer therapies can result in adverse cardiac events, including acute myocardial infarction and heart failure.
Targeting metabolic features of cancer cells might limit tumour growth and also protect the heart against adverse remodelling.
Acknowledgements
A.K. is supported by NIH award R00-HL-1417. J.M. is supported by NIH grants R01HL141466, R01HL155990 and R01HL156021. R.A.d.B. is supported by a grant from the European Research Council (ERC CoG 818715, SECRETE-HF) and by grants from the Netherlands Heart Foundation (grants 2017-21; 2017-11; 2018-30; 2020B005).
J.M. has served on advisory boards for Amgen, AstraZeneca, Audentes, Boston Biomedical, Bristol Myers Squibb, Cytokinetics, Deciphera, Immunocore, Ipsen, Janssen, Myovant, Precigen Triple-Gene, Regeneron and Takeda. R.A.d.B. reports research grants from Abbott, AstraZeneca, Boehringer Ingelheim, Cardior Pharmaceuticals, Ionis Pharmaceuticals, Novo Nordisk and Roche; and reports speaker fees from Abbott, AstraZeneca, Bayer, Novartis and Roche.
Footnotes
Peer review information
Nature Reviews Cardiology thanks Dimitrios Farmakis, Giorgio Minotti and Radek Pudil for their contribution to the peer review of this work.
Competing interests
A.K. declares no competing interests.
References
- 1.Moslehi JJ Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med 375, 1457–1467 (2016). [DOI] [PubMed] [Google Scholar]
- 2.de Boer RA et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail 22, 2272–2289 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aboumsallem JP, Moslehi J. & de Boer RA Reverse cardio-oncology: cancer development in patients with cardiovascular disease. J. Am. Heart Assoc 9, e013754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlstaedt A. et al. Oncometabolite d-2-hydroxyglutarate impairs a-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc. Natl Acad. Sci. USA 113, 10436–10441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbay EA et al. d-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev. 28, 479–490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med 371, 2488–2498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijers WC & de Boer RA Common risk factors for heart failure and cancer. Cardiovasc. Res 115, 844–853 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau ES et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol 3, 48–58 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariotto AB et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol. Biomark. Prev 18, 1033–1040 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Miller KD et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin 66, 271–289 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Mulrooney DA et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan PC et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol 26, 4401–4409 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward E, DeSantis C, Robbins A, Kohler B. & Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin 64, 83–103 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Avraham S. et al. Early cardiac remodeling promotes tumor growth and metastasis. Circulation 142, 670–683 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Meijers WC et al. Heart failure stimulates tumor growth by circulating factors. Circulation 138, 678–691 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Matetic A. et al. Impact of cancer diagnosis on causes and outcomes of 5.9 million US patients with cardiovascular admissions. Int. J. Cardiol 341, 76–83 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Karlstaedt A, Barrett M, Hu R, Gammons ST & Ky B. Cardio-oncology: understanding the intersections between cardiac metabolism and cancer biology. JACC Basic. Transl. Sci 6, 705–718 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young ME et al. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul. Syst. Bio 1, 251–262 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young ME, Laws FA, Goodwin GW & Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor a is associated with contractile dysfunction in hypertrophied rat heart. J. Biol. Chem 276, 44390–44395 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Taegtmeyer H. et al. Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ. Res 118, 1659–1701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leu M. et al. Monocarboxylate transporter-1 (MCT1) protein expression in head and neck cancer affects clinical outcome. Sci. Rep 11, 4578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurista SR, Nguyen CT, Rosenzweig A, de Boer RA & Westenbrink BD Ketone bodies for the failing heart: fuels that can fix the engine? Trends Endocrinol. Metab 32, 814–826 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Yurista SR et al. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol 77, 1660–1669 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Benjamin JS et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc. Natl Acad. Sci. USA 114, 125–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman JC & Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab 25, 42–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon MV et al. Cardiac LXRa protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Mol. Med 7, 1229–1243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngoh GA, Facundo HT, Zafir A. & Jones SP O-GlcNAc signaling in the cardiovascular system. Circ. Res 107, 171–185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murashige D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaraj S, Kelly DP & Margulies KB Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 141, 1800–1812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauzier B. et al. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J. Mol. Cell Cardiol 55, 92–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H. et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonjes M. et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med 19, 901–908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faubert B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J. & DeBerardinis RJ Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 30, 434–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vander Heiden MG, Cantley LC & Thompson CB Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warburg O, Wind F. & Negelein E. The metabolism of tumors in the body. J. Gen. Physiol 8, 519–530 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppenol WH, Bounds PL & Dang CV Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Hensley CT et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaude E. & Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat. Commun 7, 13041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katzir R. et al. The landscape of tiered regulation of breast cancer cell metabolism. Sci. Rep 9, 17760 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau AN et al. Dissecting cell-type-specific metabolism in pancreatic ductal adenocarcinoma. eLife 9, e56782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Najumudeen AK et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat. Genet 53, 16–26 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Osthus RC et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem 275, 21797–21800 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Duran A. et al. The signaling adaptor p62 is an important NF-kB mediator in tumorigenesis. Cancer Cell 13, 343–354 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Gurel B. et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod. Pathol 21, 1156–1167 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mossafa H. et al. Non-Hodgkin’s lymphomas with Burkitt-like cells are associated with c-Myc amplification and poor prognosis. Leuk. Lymphoma 47, 1885–1893 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Cantley LC & Neel BG New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. USA 96, 4240–4245 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones RG et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Lee SM, Kim JH, Cho EJ & Youn HD A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differ. 16, 738–748 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Palaskas N. et al. 18F-fluorodeoxy-glucose positron emission tomography marks MYC-overexpressing human basal-like breast cancers. Cancer Res. 71, 5164–5174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yue M, Jiang J, Gao P, Liu H. & Qing G. Oncogenic MYC activates a feedforward regulatory loop promoting essential amino acid metabolism and tumorigenesis. Cell Rep. 21, 3819–3832 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Jones S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T. et al. Mutant KRAS drives metabolic reprogramming and autophagic flux in premalignant pancreatic cells. Cancer Gene Ther. 10.1038/s41417-021-00326-4 (2021). [DOI] [PMC free article] [PubMed]
- 54.Doherty JR et al. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 74, 908–920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koelwyn GJ et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med 26, 1452–1458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donath MY & Shoelson SE Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol 11, 98–107 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Stentz FB, Umpierrez GE, Cuervo R. & Kitabchi AE Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 53, 2079–2086 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Esposito K. et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAmA 289, 1799–1804 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Bellinger AM et al. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation 132, 2248–2258 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asnani A. et al. Preclinical models of cancer therapy-associated cardiovascular toxicity: a scientific statement from the American Heart Association. Circ. Res 129, e21–e34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moslehi J, Zhang Q. & Moore KJ Crosstalk between the heart and cancer: beyond drug toxicity. Circulation 142, 684–687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koelwyn GJ, Aboumsallem JP, Moore KJ & de Boer RA Reverse cardio-oncology: exploring the effects of cardiovascular disease on cancer pathogenesis. J. Mol. Cell Cardiol 163, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tocchetti CG et al. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio-oncology insights from the joint 2019 meeting of the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc. Res 116, 1820–1834 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Tini G. et al. Cancer mortality in trials of heart failure with reduced ejection fraction: a systematic review and meta-analysis. J. Am. Heart Assoc 9, e016309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Boer RA et al. A new classification of cardio-oncology syndromes. Cardiooncology 7, 24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Wit S. & de Boer RA From studying heart disease and cancer simultaneously to reverse cardio-oncology. Circulation 144, 93–95 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Koene RJ, Prizment AE, Blaes A. & Konety SH Shared risk factors in cardiovascular disease and cancer. Circulation 133, 1104–1114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Boer RA, Meijers WC, van der Meer P. & van Veldhuisen DJ Cancer and heart disease: associations and relations. Eur. J. Heart Fail 21, 1515–1525 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pfeffer TJ, Pietzsch S. & Hilfiker-Kleiner D. Common genetic predisposition for heart failure and cancer. Herz 45, 632–636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masoudkabir F. et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 263, 343–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gibson CJ et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J. Clin. Oncol 35, 1598–1605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libby P. et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J. Am. Coll. Cardiol 74, 567–577 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calvillo-Arguelles O. et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 4, 380–387 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Jaiswal S. & Ebert BL Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haring B. et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the Women’s Health Initiative. J. Am. Heart Assoc 10, e018789 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaiswal S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med 377, 111–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dorsheimer L. et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 4, 25–33 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buscarlet M. et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130, 753–762 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Honigberg MC et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation 143, 410–423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuster JJ et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang JG et al. Monocytic microparticles activate endothelial cells in an IL-1 p-dependent manner. Blood 118, 2366–2374 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bick AG et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hou HA et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood 119, 559–568 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Ridker PM et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Ridker PM et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Buckley LF & Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur. Heart J 2063–2069 (2018). [DOI] [PubMed]
- 87.Gottschlich A, Endres S. & Kobold S. Therapeutic strategies for targeting IL-1 in cancer. Cancers (Basel) 13, 477 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nidorf SM et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med 383, 1838–1847 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Silvis MJM et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: a LoDoCo2 biomarker substudy. Atherosclerosis 334, 93–100 (2021). [DOI] [PubMed] [Google Scholar]
- 90.Yan H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med 360, 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mardis ER et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med 361, 1058–1066 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dang L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koivunen P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Losman JA et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339, 1621–1625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu X. et al. 2-Hydroxyglutarate Inhibits ATP synthase and mTOR signaling. Cell Metab. 22, 508–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohanbash G. et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J. Clin. Invest 127, 1425–1437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bunse L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med 24, 1192–1203 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Kattih B. et al. IDH1/2 mutations in acute myeloid leukemia patients and risk of coronary artery disease and cardiac dysfunction-a retrospective propensity score analysis. Leukemia 35, 1301–1316 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rzem R. et al. A gene encoding a putative FAD-dependent L-2-hydroxyglutarate dehydrogenase is mutated in L-2-hydroxyglutaric aciduria. Proc. Natl Acad. Sci. USA 101, 16849–16854 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS & Jakobs C. Progress in understanding 2-hydroxyglutaric acidurias. J. Inherit. Metab. Dis 35, 571–587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kranendijk M. et al. Evidence for genetic heterogeneity in d-2-hydroxyglutaric aciduria. Hum. Mutat 31, 279–283 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Kranendijk M. et al. IDH2 mutations in patients with d-2-hydroxyglutaric aciduria. Science 330, 336 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Maher ER & Kaelin WG Jr. von Hippel-Lindau disease. Medicine 76, 381–391 (1997). [DOI] [PubMed] [Google Scholar]
- 104.Menendez-Montes I. et al. Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Dev. Cell 39, 724–739 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Smith TG et al. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 3, e290 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith TG et al. Mutation of the von Hippel-Lindau gene alters human cardiopulmonary physiology. Adv. Exp. Med. Biol 605, 51–56 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Formenti F. et al. Regulation of human metabolism by hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 107, 12722–12727 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slingo M. et al. The von Hippel-Lindau Chuvash mutation in mice alters cardiac substrate and high-energy phosphate metabolism. Am. J. Physiol. Heart Circ. Physiol 311, H759–H767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perrotta S. et al. Effects of germline VHL deficiency on growth, metabolism, and mitochondria. N. Engl. J. Med 382, 835–844 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Lei L. et al. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol. Cell Biol 28, 3790–3803 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu SJ et al. Genotype and phenotype correlation in von Hippel-Lindau disease based on alteration of the HIF-α binding site in VHL protein. Genet. Med 20, 1266–1273 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Cho H. et al. On-target efficacy of a HIF-2a antagonist in preclinical kidney cancer models. Nature 539, 107–111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choueiri TK & Kaelin WG Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med 26, 1519–1530 (2020). [DOI] [PubMed] [Google Scholar]
- 114.Jonasch E. et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N. Engl. J. Med 385, 2036–2046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghosh MC et al. Therapeutic inhibition of HIF-2a reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood 137, 2509–2519 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baysal BE et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000). [DOI] [PubMed] [Google Scholar]
- 118.Goncalves J. et al. Loss of SDHB promotes dysregulated iron homeostasis, oxidative stress, and sensitivity to ascorbate. Cancer Res. 81, 3480–3494 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Astuti D. et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet 69, 49–54 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burnichon N. et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet 19, 3011–3020 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomlinson IP et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet 30, 406–410 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Hol JA et al. Renal cell carcinoma in young FH mutation carriers: case series and review of the literature. Fam. Cancer 19, 55–63 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Letouze E. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 23, 739–752 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Davili Z, Johar S, Hughes C, Kveselis D. & Hoo J. Succinate dehydrogenase deficiency associated with dilated cardiomyopathy and ventricular noncompaction. Eur. J. Pediatr 166, 867–870 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Bourgeron T. et al. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet 11, 144–149 (1995). [DOI] [PubMed] [Google Scholar]
- 126.Reichmann H. & Angelini C. Single muscle fibre analyses in 2 brothers with succinate dehydrogenase deficiency. Eur. Neurol 34, 95–98 (1994). [DOI] [PubMed] [Google Scholar]
- 127.Rustin P. et al. The investigation of respiratory chain disorders in heart using endomyocardial biopsies. J. Inherit. Metab. Dis 16, 541–544 (1993). [DOI] [PubMed] [Google Scholar]
- 128.Ferreira CR, van Karnebeek CDM, Vockley J. & Blau N. A proposed nosology of inborn errors of metabolism. Genet. Med 21, 102–106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keating ST, van Diepen JA, Riksen NP & El-Osta A. Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia 61, 6–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Osuna-Prieto FJ et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol 20, 151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prag HA et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res 117, 1188–1201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reddy A. et al. pH-Gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valentova M, Anker SD & von Haehling S. Cardiac cachexia revisited: the role of wasting in heart failure. Heart Fail. Clin 16, 61–69 (2020). [DOI] [PubMed] [Google Scholar]
- 134.von Haehling S. & Anker SD Cachexia as a major underestimated and unmet medical need: facts and numbers. J. Cachexia Sarcopenia Muscle 1, 1–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palenik CJ & Miller CH Treating highly infectious patients in the dental office. J. Indiana Dent. Assoc 64, 11–15 (1985). [PubMed] [Google Scholar]
- 136.Argiles JM, Busquets S, Stemmler B. & Lopez-Soriano FJ Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer 14, 754–762 (2024). [DOI] [PubMed] [Google Scholar]
- 137.Baazim H, Antonio-Herrera L. & Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol 10.1038/s41577-021-00624-w (2021). [DOI] [PMC free article] [PubMed]
- 138.Ling HH et al. Clinical significance of serum glutamine level in patients with colorectal cancer. Nutrients 11, 898 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katt WP, Antonyak MA & Cerione RA Simultaneously and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol. Pharm 12, 46–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Bermudez J. et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol 20, 775–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L. & Kitsis RN Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J. Clin. Invest 131, e145186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li W. et al. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 3, 980–988 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Li W. et al. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J. Am. Coll. Cardiol 66, 1160–1178 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Sheng CC et al. 21st century cardio-oncology: identifying cardiac safety signals in the era of personalized medicine. JACC Basic. Transl. Sci 1, 386–398 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spetz J, Moslehi J. & Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr. Treat. Options Cardiovasc. Med 20, 31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suliman HB et al. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J. Clin. Invest 117, 3730–3741 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leite de Oliveira R. et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 22, 263–277 (2012). [DOI] [PubMed] [Google Scholar]
- 148.Campia U. et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation 139, e579–e602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu JR et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer contemporary meta-analyses. Arterioscler. Thromb. Vasc. Biol 40, e55–e64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cohen P, Cross D. & Janne PA Kinase drug discovery 20 years after imatinib: progress and future directions. Nat. Rev. Drug Discov 20, 551–569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Motzer RJ et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med 356, 115–124 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Rini BI et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase III JAVELIN renal 101 trial. J. Clin. Oncol 10.1200/JCO.21.01806 (2022). [DOI] [PMC free article] [PubMed]
- 153.Nazer B, Humphreys BD & Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation 124, 1687–1691 (2011). [DOI] [PubMed] [Google Scholar]
- 154.Richards CJ et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J. Clin. Oncol 29, 3450–3456 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Bair SM, Choueiri TK & Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc. Med 23, 104–113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giordano FJ et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc. Natl Acad. Sci. USA 98, 5780–5785 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee Y. et al. Life-threatening hypoglycemia induced by a tyrosine kinase inhibitor in a patient with neuroendocrine tumor: a case report. Diabetes Res. Clin. Pract 93, e68–e70 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Louvet C. et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 18895–18900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Villalta SA et al. Inhibition of VEGfR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function. Diabetes 62, 2870–2878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hamberg P. et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J. Clin. Oncol 24, e30–e31 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Barber MC, Mauro MJ & Moslehi J. Cardiovascular care of patients with chronic myeloid leukemia (CML) on tyrosine kinase inhibitor (TKI) therapy. Hematol. Am. Soc. Hematol. Educ. Program 2017, 110–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manouchehri A. et al. Tyrosine kinase inhibitors in leukemia and cardiovascular events: from mechanism to patient care. Arterioscler. Thromb. Vasc. Biol 40, 301–308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moslehi JJ & Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J. Clin. Oncol 33, 4210–4218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Veneri D, Franchini M. & Bonora E. Imatinib and regression of type 2 diabetes. N. Engl. J. Med 352, 1049–1050 (2005). [DOI] [PubMed] [Google Scholar]
- 165.Uraizee I, Cheng S. & Moslehi J. Reversible cardiomyopathy associated with sunitinib and sorafenib. N. Engl. J. Med 365, 1649–1650 (2011). [DOI] [PubMed] [Google Scholar]
- 166.Chintalgattu V. et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci. Transl. Med 5, 187ra169 (2013). [DOI] [PMC free article] [PubMed]
- 167.May D. et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc. Natl Acad. Sci. USA 105, 282–287 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moslehi J. et al. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation 122, 1004–1016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bekeredjian R. et al. Conditional HIF-1α expression produces a reversible cardiomyopathy. PLoS ONE 5, e11693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yen K. et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 7, 478–493 (2017). [DOI] [PubMed] [Google Scholar]
- 171.DiNardo CD et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med 378, 2386–2398 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Gatto L. et al. IDH inhibitors and beyond: the cornerstone of targeted glioma treatment. Mol. Diagn. Ther 25, 457–473 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Kam AE, Masood A. & Shroff RT Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol 6, 956–969 (2021). [DOI] [PubMed] [Google Scholar]
- 174.Norsworthy KJ et al. FDA approval summary: ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-1 mutation. Clin. Cancer Res 25, 3205–3209 (2019). [DOI] [PubMed] [Google Scholar]
- 175.Baik AH et al. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ. Res 128, 1780–1801 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lehmann LH et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol. 6, 1329–1337 (2021). [DOI] [PubMed] [Google Scholar]
- 177.Johnson DB et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 21, e398–e404 (2020). [DOI] [PubMed] [Google Scholar]
- 178.Hu JR et al. High sensitivity troponin T and NT-proBNP in patients receiving chimeric antigen receptor (CAR) T-cell therapy. Clin. Hematol. Int 3, 96–102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dolladille C. et al. Chimeric antigen receptor T-cells safety: a pharmacovigilance and meta-analysis study. Am. J. Hematol 96, 1101–1111 (2021). [DOI] [PubMed]
- 180.Salem JE, Ederhy S, Lebrun-Vignes B. & Moslehi JJ Cardiac events associated with chimeric antigen receptor T-cells (CAR-T): a VigiBase perspective. J. Am. Coll. Cardiol 75, 2521–2523 (2020). [DOI] [PubMed] [Google Scholar]
- 181.Abou-Alfa GK et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tap WD et al. Phase I study of the mutant IDH1 inhibitor ivosidenib: safety and clinical activity in patients with advanced chondrosarcoma. J. Clin. Oncol 38, 1693–1701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Descamps O, Riondel J, Ducros V. & Roussel AM Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech. Ageing Dev 126, 1185–1191 (2005). [DOI] [PubMed] [Google Scholar]
- 184.Berrigan D, Perkins SN, Haines DC & Hursting SD Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 23, 817–822 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Mattson MP, Longo VD & Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev 39, 46–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Longo VD & Mattson MP Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de Cabo R. & Mattson MP Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med 381, 2541–2551 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Lien EC et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599, 302–307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stroud AM et al. Association between weight loss and serum biomarkers with risk of incident cancer in the Longitudinal Assessment of Bariatric Surgery cohort. Surg. Obes. Relat. Dis 16, 1086–1094 (2020). [DOI] [PubMed] [Google Scholar]
- 190.Dale KM, Coleman CI, Henyan NN, Kluger J. & White CM Statins and cancer risk: a meta-analysis. JAMA 295, 74–80 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Rugo HS et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Ann. Oncol 31, 1001–1010 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Andre F. et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann. Oncol 32, 208–217 (2021). [DOI] [PubMed] [Google Scholar]
- 193.Ferraro GB et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wei X. et al. Targeting ACLY attenuates tumor growth and acquired cisplatin resistance in ovarian cancer by inhibiting the PI3K-AKT pathway and activating the AMPK-ROS pathway. Front. Oncol 11, 642229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xin M. et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 7, 44252–44265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tracz-Gaszewska Z. & Dobrzyn P. Stearoyl-CoA desaturase 1 as a therapeutic target for the treatment of cancer. Cancers 11, 948 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]