Abstract
Background
To date, more than 761 million confirmed SARS-CoV-2 infections have been recorded globally, and more than half of all children are estimated to be seropositive. Despite high SARS-CoV-2 infection incidences, the rate of severe COVID-19 in children is low. We aimed to assess the safety and efficacy or effectiveness of COVID-19 vaccines approved in the EU for children aged 5–11 years.
Methods
In this systematic review and meta-analysis, we included studies of any design identified through searching the COVID-19 L·OVE (living overview of evidence) platform up to Jan 23, 2023. We included studies with participants aged 5–11 years, with any COVID-19 vaccine approved by the European Medicines Agency—ie, mRNA vaccines BNT162b2 (Pfizer-BioNTech), BNT162b2 Bivalent (against original strain and omicron [BA.4 or BA.5]), mRNA-1273 (Moderna), or mRNA-1273.214 (against original strain and omicron BA.1). Efficacy and effectiveness outcomes were SARS-CoV-2 infection (PCR-confirmed or antigen-test confirmed), symptomatic COVID-19, hospital admission due to COVID-19, COVID-19-related mortality, multisystem inflammatory syndrome in children (MIS-C), and long-term effects of COVID-19 (long COVID or post-COVID-19 condition as defined by study investigators or per WHO definition). Safety outcomes of interest were serious adverse events, adverse events of special interest (eg, myocarditis), solicited local and systemic events, and unsolicited adverse events. We assessed risk of bias and rated the certainty of evidence (CoE) using the Grading of Recommendations Assessment, Development and Evaluation approach. This study was prospectively registered with PROSPERO, CRD42022306822.
Findings
Of 5272 screened records, we included 51 (1·0%) studies (n=17 [33%] in quantitative synthesis). Vaccine effectiveness after two doses against omicron infections was 41·6% (95% CI 28·1–52·6; eight non-randomised studies of interventions [NRSIs]; CoE low), 36·2% (21·5–48·2; six NRSIs; CoE low) against symptomatic COVID-19, 75·3% (68·0–81·0; six NRSIs; CoE moderate) against COVID-19-related hospitalisations, and 78% (48–90, one NRSI; CoE very low) against MIS-C. Vaccine effectiveness against COVID-19-related mortality was not estimable. Crude event rates for deaths in unvaccinated children were less than one case per 100 000 children, and no events were reported for vaccinated children (four NRSIs; CoE low). No study on vaccine effectiveness against long-term effects was identified. Vaccine effectiveness after three doses was 55% (50–60; one NRSI; CoE moderate) against omicron infections, and 61% (55–67; one NRSI; CoE moderate) against symptomatic COVID-19. No study reported vaccine efficacy or effectiveness against hospitalisation following a third dose. Safety data suggested no increased risk of serious adverse events (risk ratio [RR] 0·83 [95% CI 0·21–3·33]; two randomised controlled trials; CoE low), with approximately 0·23–1·2 events per 100 000 administered vaccines reported in real-life observations. Evidence on the risk of myocarditis was uncertain (RR 4·6 [0·1–156·1]; one NRSI; CoE low), with 0·13–1·04 observed events per 100 000 administered vaccines. The risk of solicited local reactions was 2·07 (1·80–2·39; two RCTs; CoE moderate) after one dose and 2·06 (1·70–2·49; two RCTs; CoE moderate) after two doses. The risk of solicited systemic reactions was 1·09 (1·04–1·16; two RCTs; CoE moderate) after one dose and 1·49 (1·34–1·65; two RCTs; CoE moderate) after two doses. The risk of unsolicited adverse events after two doses (RR 1·21 [1·07–1·38]; CoE moderate) was higher among mRNA-vaccinated compared with unvaccinated children.
Interpretation
In children aged 5–11 years, mRNA vaccines are moderately effective against infections with the omicron variant, but probably protect well against COVID-19 hospitalisations. Vaccines were reactogenic but probably safe. Findings of this systematic review can serve as a basis for public health policy and individual decision making on COVID-19 vaccination in children aged 5–11 years.
Funding
German Federal Joint Committee.
Research in context.
Evidence before this study
Although high rates of SARS-CoV-2 infections have been observed in children, the rate of severe COVID-19 cases has been low and appears to be even lower with the spread of the omicron variant. However, in rare cases, SARS-CoV-2 infection in children can lead to severe diseases, including multisystem inflammatory syndrome (also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [PIMS-TS]) associated with COVID-19. As of November, 2021, the first COVID-19 vaccine (BNT162b2 [Pfizer–BioNTech]) has been licensed in the EU for use in children aged 5–11 years. Assessment of the safety, efficacy, and immunogenicity of the COVID-19 vaccines currently approved by the European Medicines Agency (EMA; BNT162b2 and mRNA-1273 [Moderna]) in this population is necessary to inform and support the development of vaccination recommendations of National Immunization Technical Advisory Groups and other immunisation policy makers. We searched the COVID-19 LOVE (living overview of evidence) literature database using keywords related to “vaccination” and “immunization” in combination with terms related to “children”. We included randomised controlled trials and non-randomised studies of interventions assessing vaccine efficacy or effectiveness, immunogenicity, and safety, as well as single-arm observational studies of vaccine safety, published by Jan 23, 2023, without any language restrictions. Studies on children aged 5–11 years who had received at least one dose of an EMA-approved COVID-19 vaccine were eligible. We assessed risk of bias using standard tools (the revised Cochrane risk of bias tool for randomised trials, the Risk of Bias in Non-Randomized Studies of interventions tool, and the Quality In Prognosis Studies tool) and the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation approach on an outcome level.
Added value of this study
This systematic review on mRNA vaccines for children aged 5–11 years identified relevant evidence for assessing vaccine efficacy or effectiveness and safety, which serves as a base for clinical and public health decision making. We showed that mRNA vaccines are effective, but also that protective effectiveness of a primary vaccination series is insufficient to reliably prevent infections with the omicron variant (41·6% [95% CI 28·1–52·6] for two doses). However, data from the first published study on a third dose showed that vaccine efficacy or effectiveness could be increased through a third booster dose (55% [50–60] for three doses).
Implications of all the available evidence
Given the heterogeneous findings on vaccine effectiveness, the established reactogenicity, and remaining uncertainties regarding serious adverse events and myocarditis, this systematic review adds value to previous evidence and might help guide clinical and public health discussions on the need for and importance of COVID-19 vaccination in children, as well as individual decision making. We highlight the need for continuous re-evaluation of the evidence with particular attention to the changing epidemiological landscape (eg, incidences or emerging variants) to support policy makers, medical guidelines, and clinical decisions to ensure children's health in the best possible way.
Introduction
Since the emergence of SARS-CoV-2, more than 762 million confirmed cases of COVID-19 and 6·9 million associated deaths have been recorded globally up to April 12, 2023. In December, 2020, the first COVID-19 vaccine was authorised in the EU.1 The introduction of COVID-19 vaccines was a success, with an estimated 14 million COVID-19-associated deaths being prevented over the first year of vaccination programmes worldwide.2 Surveillance data from Germany showed that children aged 5–14 years had the highest SARS-CoV-2 incidence rates across all age groups in 2022.3 A systematic review on seroprevalence showed that by April, 2022, approximately 57% of children were seropositive worldwide.4 Both findings highlight the susceptibility and need for protection in these age groups, given that children aged 10 years or younger account for nearly 20% of the global population.5 In November, 2021, the first COVID-19 vaccine (BNT162b2 [Pfizer–BioNTech]) was also approved for children aged 5–11 years by the European Medicines Agency (EMA),6 and in October, 2022, COVID-19 vaccines (BNT162b2 and mRNA-1273) were approved by the EMA for use in younger children from age 6 months.
Early in the pandemic, data indicated that children are at low risk of severe COVID-19. However, SARS-CoV-2 infection can cause severe medical conditions, including multisystem inflammatory syndrome in children (MIS-C; also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [PIMS-TS]).7, 8 Severity of COVID-19, including risk of developing MIS-C, varies with the SARS-CoV-2 variant of infection. Since the latest shift in epidemiology from delta (B.1.617.2) to omicron (B.1.1.529), evidence suggests even lower odds of moderate or severe COVID-19 (odds ratio [OR] 0·47 [95% CI 0·33–0·66]) when comparing omicron with delta variant infections in children aged 6–11 years.9 Even though the omicron variant is associated with higher transmissibility, cases of MIS-C were rarely reported.10 Thus, given the low risk of severe disease in healthy children following SARS-CoV-2 infection, a careful assessment of the benefits and risks of COVID-19 vaccination is needed.
The aim of this systematic review was to inform National Immunization Technical Advisory Groups and other immunisation policy makers. Our objectives were to assess the safety and efficacy or effectiveness of vaccines against COVID-19 approved in the EU for children aged 5–11 years.
Methods
Search strategy and selection criteria
This systematic review is reported according to the PRISMA 2020 reporting guideline.11 Our primary literature database was the COVID-19 L·OVE (living overview of evidence) platform. We searched for articles published up to Jan 23, 2023, using search terms related to “vaccination” and “immunization” combined with terms related to “children” (appendix 1 pp 1–2). We identified duplicate references using Systematic Review Accelerator12 and imported references into Covidence for screening.
We included randomised controlled trials (RCTs) and non-randomised studies of interventions (NRSIs) for assessing vaccine efficacy or effectiveness, immunogenicity, and safety. We additionally included observational (single-arm) studies to assess vaccine safety. Participants had to be aged 5–11 years.
We included any vaccines specifically designed to prevent COVID-19, which had been approved by the EMA. Up to Jan 23, 2023, those vaccines were BNT162b2 (5–11-year-old children, two doses 10 μg, 21 days apart for the primary vaccination series, or an additional booster dose 10 μg at least 6 months after the second dose), BNT162b2 bivalent (against original strain and omicron BA.4 and BA.5; booster dose 10 μg, at least 6 months after the second dose of the primary vaccination series), mRNA-1273 (Moderna; 6–11-year-old children, two doses 50 μg, 28 days apart for the primary vaccination series, or an additional booster dose 50 μg at least 3 months after the second dose), and mRNA-1273.214 (Moderna; against original strain and omicron BA.1; booster dose 50 μg, at least 3 months after the second dose of the primary vaccination series). Complete and incomplete primary vaccination series were eligible, including heterologous prime–boost vaccinations as well as booster vaccinations. The intervention was compared with placebo, no vaccination, other COVID-19 vaccines, or vaccination schedules that differed from initial approval (eg, heterologous vaccination with different vaccine types, lower or higher dosage, and shortened or extended time intervals between doses).
Two reviewers (VP, WS, or IT) independently screened titles and abstracts. In case of disagreement, the record was passed on to full-text review. The full texts were obtained and assessed independently for relevance by two reviewers (VP, WS, or IT). Disagreements were discussed and resolved. If necessary, two additional reviewers were consulted for the final decision (JJM, TH). After checking the full texts for eligibility, a list of included studies, ongoing studies, studies awaiting classification, and excluded studies was generated using EndNote (version 20; appendix 1 pp 7–24).
Two reviewers (VP, WS, or IT) independently extracted data on study and participant characteristics. Disagreements were resolved by jointly reviewing the data source.
Ethics approval was not required as this systematic review is based exclusively on published literature.
Outcomes
Efficacy and effectiveness outcomes were SARS-CoV-2 infection (PCR-confirmed or antigen-test confirmed), symptomatic COVID-19, hospital admission due to COVID-19, COVID-19-related mortality, MIS-C, long-term effects of COVID-19 (long COVID or post-COVID-19 condition as defined by study investigators or per WHO definition [ie, experiencing one or more symptoms, which cannot be explained by alternative diagnoses and generally affect everyday functioning for at least 2 months in individuals with a confirmed or probable previous SARS-CoV-2 infection]13). Safety outcomes of interest were serious adverse events, adverse events of special interest (eg, myocarditis), reactogenicity, solicited local and systemic events (solicited adverse events), and unsolicited adverse events. Additional outcomes were immunogenicity parameters (neutralising antibody titres, IgG, and T-cell response) and or intensive care unit (ICU) admission due to COVID-19.
Data appraisal and synthesis
To assess risk of bias, we used the revised Cochrane risk of bias tool for randomised trials (RoB 2.0 tool),14 the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool,15 and adapted the Quality in Prognosis Studies (QUIPS) tool for single-arm studies.16 We excluded outcomes rated with ROBINS-I as critical from the data synthesis to avoid misleading conclusions (appendix 1 p 3).15 For the use of QUIPS, we considered the event of vaccination as prognostic factor (appendix 1 p 4).
We summarised the certainty of evidence (CoE) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, resulting in the overall rating of high, moderate, low, or very low for each assessed outcome (appendix 1 p 6).17 GRADE Working Group grades of evidence (ranging from high to very low certainty) are explained in appendix 1 (p 39). In accordance with the GRADE guidelines on rating the CoE for NRSIs, we started with a high CoE for outcomes assessed with ROBINS-I.18
We calculated vaccine efficacy or effectiveness and their 95% CIs using the vaccine effect ratio as reported by the authors of studies—eg, adjusted (NRSIs) or unadjusted (RCTs) OR, risk ratio (RR), hazard ratio, or incidence rate ratio as follows: vaccine efficacy or effectiveness = (1 – vaccine effect ratio) × 100. Vaccine efficacy or effectiveness estimates were expressed as percentages with their corresponding 95% CIs, where values greater than 0% suggest a protective effect of the vaccine.
We did meta-analyses separately for RCTs and NRSIs using a random effects model for the primary analysis. We used the restricted maximum likelihood method for estimating the between-study variance19 and applied the Hartung-Knapp adjustment for random effects meta-analyses20, 21, 22 with three or more studies. We did not perform meta-analysis for single-arm studies, but provide results in summary tables instead. We used R (version 4.2.1) for all analyses using the package meta. 23
Details on data analysis, subgroup, and sensitivity analyses are provided in appendix 1 (p 5). Because of missing data, we did not do any tests for subgroup differences. Subgroup investigations were exploratory instead. This study was prospectively registered with PROSPERO, CRD42022306822.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
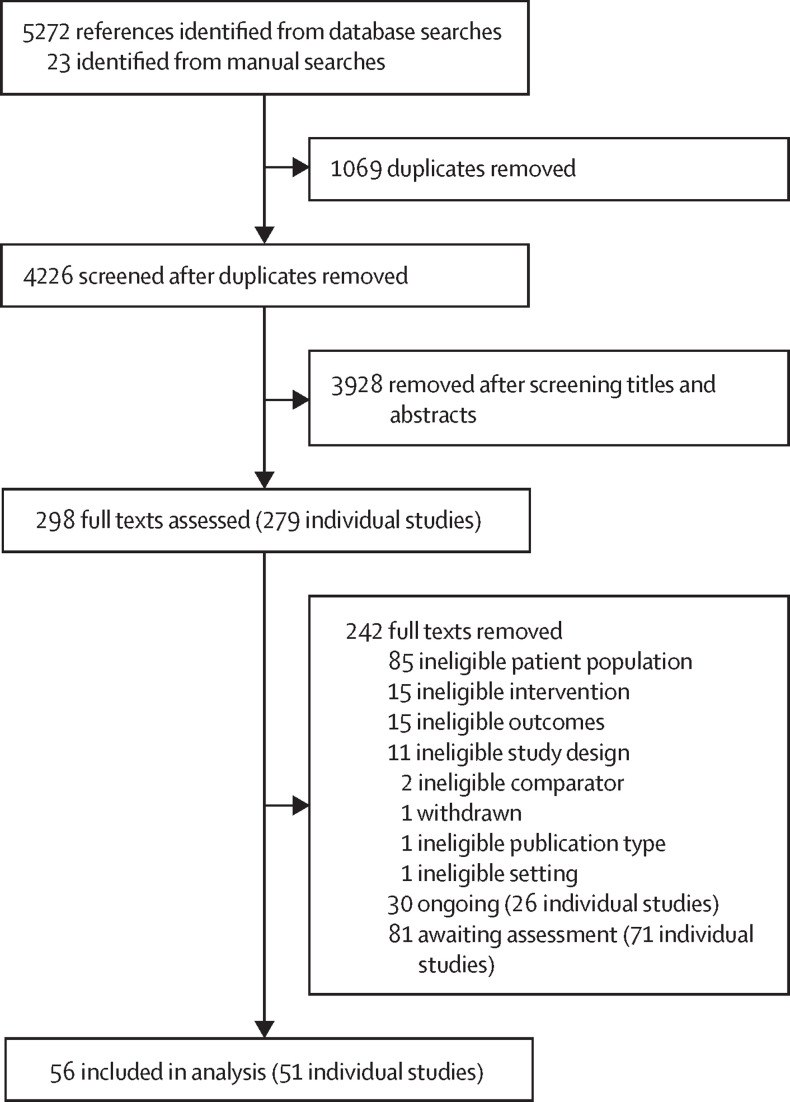
We identified 5272 references through database searches and an additional 23 references through manual searches up to Jan 23, 2023. After removal of duplicates, we screened 4226 (80·2%) records and assessed 298 (6·6%) full texts (figure 1 ). Overall, we excluded 242 full texts, reporting on 228 studies (26 [11·4%] ongoing studies, 71 [31·1%] monitored for further eligibility assessments, and 131 [57·5%] studies that did not meet eligibility criteria; appendix 1 pp 11–24). Studies for further monitoring comprised ongoing and completed studies on COVID-19 vaccines in children younger than 12 years that are not yet approved by the EMA. Finally, we included 51 studies in the systematic review and 17 of those in the meta-analysis (appendix 1 pp 7–24).
Figure 1.
PRISMA flow diagram
Of the 51 studies included, four (7·8%) were RCTs for the mRNA-based vaccines BNT162b224 and mRNA-1273.25 RCTs reported results for participants included in phase 1 of the trial and phase 2/3 separately. Other studies were NRSIs with a retrospective (n=28 [55%]) or prospective study design (n=8 [16%]), surveillance studies (n=9 [18%]), ecological study (n=1 [2%]), or case series (n=1 [2%]). 18 (35%) studies were not yet peer reviewed, and either published on preprint servers, the Morbidity and Mortality Weekly Report provided by the US Centers for Disease Control and Prevention, or in another format of short communication (eg, letters).
The number of overall assessed participants could not be estimated because studies partially reported on number of administered vaccines or person-days at risk instead of number of participants. Included data records ranged from 12 to more than 3·4 million participants.26, 27 Studies were primarily done in high-income countries (eg, the USA, Canada, and Israel), and assessed a complete primary schedule of BNT162b2 vaccine (n=46 [90%]). Three (6%) studies only assessed the effect of a single dose of either BNT162b2 or mRNA-1273,28, 29, 30 and nine (18%) studies assessed the effect of a monovalent31, 32, 33, 34, 35, 36, 37, 38 or bivalent (BNT162b2 bivalent [against original strain or omicron BA.4 or BA.5])39 booster vaccination (appendix 1 pp 26–33). Participants' age was in line with the authorised age group (ie, 5–11 years for studies on BNT162b2 or BNT162b2 bivalent and 6–11 years for studies on mRNA-1273; appendix 1 pp 26–33). Sex distribution was well balanced in most studies (appendix 1 pp 26–33). Little information was available on comorbidities and serological status (appendix 1 pp 26–33).
Overall, we had at least some concerns of bias for all efficacy or effectiveness outcomes. Further, most safety outcomes were assessed with at least some concerns of bias. As recommended in the ROBINS-I guidance, outcomes rated with a critical risk of bias were not included in data synthesis.15 Excluded outcomes were SARS-CoV-2 infections in one study,36 COVID-19-related hospitalisation in three studies,27, 40, 41 ICU admissions in two studies,27, 41 deaths reported in one study,41 and myocarditis in one study (appendix 1 pp 34–36; appendix 2).35
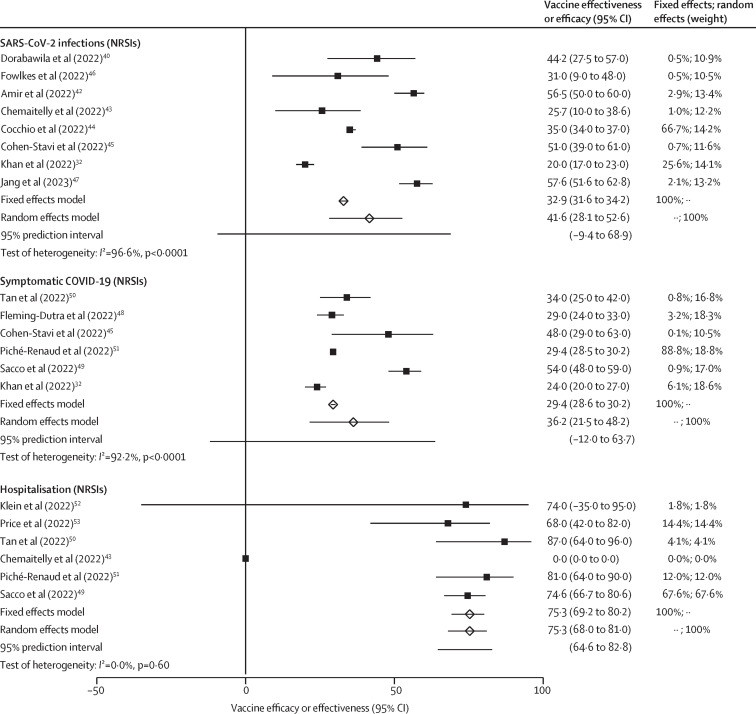
Vaccine effectiveness against SARS-CoV-2 infections with the omicron variant after two doses was 41·6% (95% CI 28·1–52·6; eight NRSIs; CoE low; table ; figure 2 ). Five of the NRSIs assessed vaccine effectiveness over time.40, 43, 44, 47 All reported a substantial reduction in vaccine effectiveness against SARS-CoV-2 infections of at least 15% from the timepoint of first measurement after two-dose vaccination to the timepoint of last measurement (appendix 1 p 40). One study32 reported vaccine effectiveness against omicron infections after the third dose (vaccine effectiveness 55% [50–60]) and reported no effect of waning protection up to 4 months after the booster.
Table.
Summary of findings
| Vaccine doses, n | Absolute effect*with placebo or no vaccination | Absolute effect*with vaccination (95% CI) | Relative effect†(95% CI) | Timing of outcome measurement | Participants, n | Certainty of the evidence (GRADE) | Interpretation | ||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine effectiveness | |||||||||
| SARS-CoV-2 infection (PCR-confirmed or antigen-test confirmed) | |||||||||
| NRSI | 2 | 20 793 per 100 000 | 8650 per 100 000 (5843 to 10 937) | VE 41·6% (28·1–52·6); VE ratio 0·584 (0·474–0·739) | ≥14 days after second dose to median of 4 months after second dose | >3 376 000‡32, 40, 42, 43, 44, 45, 46, 47 | Low; downgraded by two levels for serious inconsistency (I2=96·6%) | Primary vaccination series probably slightly reduces the risk of SARS-CoV-2 infections with omicron. | |
| NRSI | 3 | 27 595 per 100 000 | 12 418 per 100 000 (11 038 to 13 798) | VE 55% (50–60); VE ratio 0·45 (0·40–0·50) | Up to ≥3 months after third dose | 60 57432 | Moderate; downgraded by one level for serious imprecision (one study only) | Booster vaccination probably reduces the risk of SARS-CoV-2 infections with omicron. | |
| Symptomatic COVID-19 | |||||||||
| NRSI | 2 | 31 326 per 100 000 | 19 203 per 100 000 (15 005 to 24 528) | VE 36·2% (21·5–48·2); VE ratio 0·638 (0·518–0·749) | ≥7 days after second dose to median of 4 months after second dose | 3 262 72732, 45, 48, 49, 50, 51 | Low; downgraded by two levels for serious inconsistency (I2=92·2%) | Primary vaccination series probably slightly reduces the risk of symptomatic COVID-19. | |
| NRSI | 3 | NR§ | NE | VE 61% (55–67); VE ratio 0·39 (0·33–0·45) | Up to ≥3 months after third dose | 60 57432 | Moderate; downgraded by one level for serious imprecision (one study only) | Booster vaccination probably reduces the risk of symptomatic COVID-19. | |
| Hospitalisation due to COVID-19 | |||||||||
| NRSI | 2 | 47 per 100 000¶ | 12 per 100 000 (9 to 15) | VE 75·3% (68·0–81·0); VE ratio 0·25 (0·19–0·32) | ≥7 days after second dose to median of 71 days after second dose | 3 058 48043, 49, 50, 51, 52, 53 | Moderate; downgraded by one level for risk of bias (two studies with a serious risk and four studies with a moderate risk) | Primary vaccination series probably reduces the risk of hospitalisation due to omicron variant-induced COVID-19. | |
| NRSI | 3 | NA | NA | VE NA | NA | 0 | NA | Outcome was not reported in any study. | |
| COVID-19 related mortality | |||||||||
| NRSI | 2 | <1 per 100 000‖ | NE, 0 cases observed | VE NE, 0 cases in vaccinated group, 1 case in control group | Median of 34 days from vaccination to hospitalisation | 2 869 87441, 43, 49, 53 | Low; downgraded by two levels for very serious imprecision (zero or few events) | The evidence is uncertain about the effect of a primary vaccination series on the risk of mortality due to omicron variant-induced COVID-19. | |
| NRSI | 3 | NA | NA | VE NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Multisystem inflammatory syndrome in children | |||||||||
| NRSI | 2 | 18 695 per 100 000** | 4113 per 100 000 (1870 to 9721) | VE 78% (48–90); OR 0·22 (0·10–0·52) | NA | 37454 | Very low; downgraded by one level for serious imprecision (one study only), one level for risk of bias (one study with a serious risk), and one level for serious indirectness (hospitalised cases only) | The evidence is very uncertain about the effect of a primary vaccination series on the risk of PIMS-TS due to infections with omicron. | |
| NRSI | 3 | NA | NA | VE NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Long-term effects of COVID-19 (long COVID or post-COVID-19 condition) | |||||||||
| NRSI | NA | NA | NA | VE NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Vaccine safety | |||||||||
| Serious adverse events | |||||||||
| RCT | 2 | 172 per 100 000 | 143 per 100 000 (36 to 572) | RR 0·83 (0·21–3·33) | Up to median 50–70 days after second dose | 627024, 25 | Low; downgraded by two levels for very serious imprecision (few events and very wide CIs) | The evidence is uncertain about the effect of EMA-approved COVID-19 mRNA vaccines on the risk of serious adverse events. | |
| RCT | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| NRSI | NA | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Adverse events of special interest (myocarditis) | |||||||||
| RCT | 2 | NE, 0 cases observed | NE, 0 cases observed | RR NE, 0 cases observed | Up to median 50–70 days after second dose | 624424, 25 | Low; downgraded by two levels for very serious imprecision (zero events) | The evidence is uncertain about the effect of EMA-approved COVID-19 mRNA vaccines on the risk of myocarditis. | |
| RCT | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| NRSI | 2 | 2 per 100 000 | 10 per 100 000 (0 to 324) | RR 4·6 (0·1–156·1) | 97 days after second dose | 641 57257 | Very low; downgraded by one level for serious indirectness (hospitalised cases only) and two levels for very serious imprecision (few events and very wide CIs) | The evidence is very uncertain about the effect of EMA-approved COVID-19 mRNA vaccines on the risk of myocarditis. | |
| NRSI | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Reactogenicity, local events (solicited adverse events) | |||||||||
| RCT | 1 | 42 169 per 100 000 | 87 289 per 100 000 (75 904 to 100 000) | RR 2·07 (1·80–2·39) | 7 days after dose | 625924, 25 | Moderate; downgraded by one level for risk of bias (two studies with some concerns of bias) | EMA-approved COVID-19 mRNA vaccines probably increase the risk of local reactions after the first vaccine dose. | |
| RCT | 2 | 42 598 per 100 000 | 87 752 per 100 000 (72 417 to 100 000) | RR 2·06 (1·70–2·49) | 7 days after dose | 619624, 25 | Moderate; downgraded by one level for risk of bias (two studies with some concerns of bias) | EMA-approved COVID-19 mRNA vaccines probably increase the risk of local reactions after the second vaccine dose. | |
| RCT | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| NRSI | NA | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Reactogenicity, systemic events (solicited adverse events) | |||||||||
| RCT | 1 | 48 939 per 100 000 | 53 343 per 100 000 (50 896 to 56 769) | RR 1·09 (1·04–1·16) | 7 days after dose | 625924, 25 | Moderate; downgraded by one level for risk of bias (two studies with some concerns of bias) | EMA-approved COVID-19 mRNA vaccines probably slightly increase the risk of systemic reactions after the first vaccine dose. | |
| RCT | 2 | 44 295 per 100 000 | 65 999 per 100 000 (59 355 to 73 087) | RR 1·49 (1·34–1·65) | 7 days after dose | 619624, 25 | Moderate; downgraded by one level for risk of bias (two studies with some concerns of bias) | EMA-approved COVID-19 mRNA vaccines probably increase the risk of local reactions after the second vaccine dose. | |
| RCT | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| NRSI | NA | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| Unsolicited adverse events | |||||||||
| RCT | 2 | 15 072 per 100 000 | 18 237 per 100 000 (16 127 to 20 799) | RR 1·21 (1·07–1·38) | 1 month or 28 days after second dose | 627024, 25 | Moderate; downgraded by one level for risk of bias (two studies with some concerns of bias) | EMA-approved COVID-19 mRNA vaccines probably increase the risk of unsolicited adverse events. | |
| RCT | 3 | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
| NRSI | NA | NA | NA | RR NA | NA | 0 | NA | Outcome was not reported in any study. | |
EMA=European Medicines Agency. GRADE=Grading of Recommendations, Assessment, Development and Evaluation. NA=not applicable. NE=not estimable. NR=not reported. NRSI=non-randomised study of intervention. PIMS-TS=paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. RCT=randomised controlled trial. RR=risk ratio. VoC=variant of concern.
The estimated absolute effect refers to the difference between the observed baseline risk reported for the unvaccinated control group and the risk for experiencing an outcome after vaccination. The absolute effect estimated for the intervention group is based on the relative effect magnitude of an effect and the baseline risk—ie, (observed risk/100 000 unvaccinated children) × relative effect.
Relative effects (vaccine effectiveness or RRs were derived from meta-analysis, or from one study if no pooled estimate was available).
Two (25%) of eight studies did not report the number of participants. The studies reporting the number of participants included 3 376 655 children.
Crude number of symptomatic COVID-19 cases in unvaccinated children not reported.
Baseline risk was partially driven from case control studies and does not reflect the true incidence risk in this age group.
0 deaths in 1 081 881 vaccinated versus three deaths in 1 787 993 unvaccinated children observed.
Hospitalised cases only were included in the analysis; baseline risk was driven by case control studies, thus does not reflect the true incidence risk in age group.
Figure 2.
Vaccine effectiveness outcomes against omicron
A vaccine effectiveness estimate greater than 0% favours mRNA vaccines. NRSI=non-randomised study of intervention.
Vaccine effectiveness of EMA-approved mRNA COVID-19 vaccines against symptomatic COVID-19 was 36·2% (95% CI 21·5–48·2; n=3 262 727; six NRSIs; CoE low) after the emergence of the omicron variant (figure 2; table). Three studies assessed vaccine effectiveness over time and also found a reduction of vaccine effectiveness against symptomatic COVID-19 from the timepoint of first measurement of the second dose to the timepoint of last measurement of at least 8% (appendix 1 p 40).49, 51 Vaccine effectiveness against symptomatic COVID-19 could be restored to higher levels after the third dose (61% [55–67]).32
In observational studies (omicron era), vaccine effectiveness against hospitalisations due to COVID-19 was 75·3% (95% CI 68·0–81·0; n=3 058 480; six NRSIs; CoE moderate; figure 2; table). The outcome was rated with a critical risk of bias in three studies,27, 40, 41 which were therefore excluded from analysis.
Of four NRSIs reporting COVID-19-related mortality, one event occurred before discharge from hospital in a non-vaccinated participant of a test-negative case-control study (n=70 two-dose-vaccinated and n=467 unvaccinated)53 and two deaths in non-vaccinated participants of a cohort study (n=1 063 035 two-dose-vaccinated and n=1 768 497 unvaccinated;49 table). Adjusted effect estimates were not available.
A test-negative case-control study, including 374 children admitted to hospital with COVID-19,54 reported a decreased risk of MIS-C for two-dose-vaccinated children (vaccine effectiveness 78% [95% CI 48–90]; CoE very low; table).
We identified no data on the effect of COVID-19 vaccination on long-term effects of COVID-19 condition (table). Across all investigated outcomes, a protective effect after one vaccine dose was shown, but effectiveness was substantially lower than after two or three doses (appendix 1 pp 37–41). With regard to the SARS-CoV-2 variant, vaccine efficacy or effectiveness against pre-omicron variants was higher for all investigated outcomes with available data (ie, SARS-CoV-2 infections and symptomatic COVID-19) than against omicron-induced infections (appendix 1 pp 37–41).
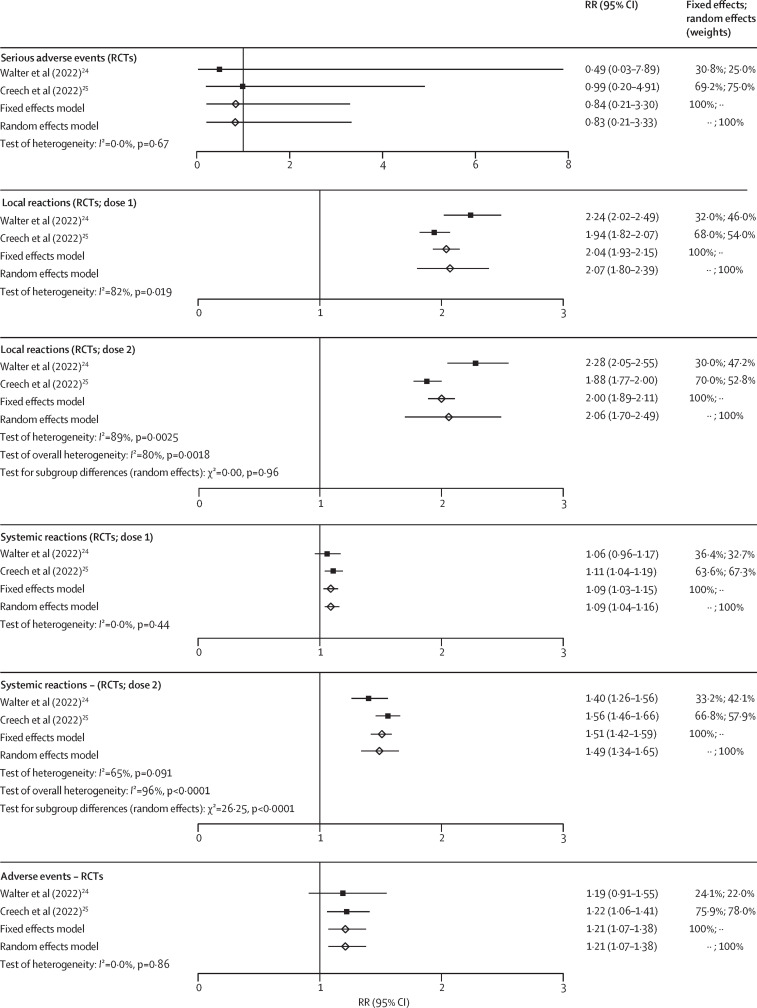
Serious adverse events were reported in both phase 2/3 RCTs.24, 25 Evidence suggests no increased risk when receiving a primary vaccination series (RR 0·83 [95% CI 0·21–3·33]; n=6270; CoE low; figure 3 ; table). A population-wide surveillance study from the USA reported an event rate of approximately 1·2 serious adverse events per 100 000 administered vaccines after primary vaccination and 0·23–0·46 serious adverse events per 100 000 booster vaccinations.33, 39, 55 Few events were reported in phase 1 trials and observational studies (appendix 1 p 42).
Figure 3.
Vaccine safety outcomes
RR greater than 1 favours placebo. RCT=randomised controlled trial. RR=risk ratio.
Regarding adverse events of special interest, no cases of myocarditis were observed in the two phase 2/3 RCTs (table). The evidence on the risk for myocarditis was uncertain and did not indicate an increased risk on the basis of one population-wide prospective cohort study (RR 4·6 [95% CI 0·1–156·1]).56 Data from a US vaccine safety surveillance study showed a rate of 0·13–1·04 myocarditis events per 100 000 administered vaccines after primary vaccination (appendix 1 pp 42–43).55 No myocarditis events were observed after booster vaccinations (appendix 1 pp 42–43).33, 39
In terms of reactogenicity, solicited local events were assessed in both phase 2/3 RCTs24, 25 and were more frequent in vaccinated children than in unvaccinated children after the first dose (RR 2·07 [95% CI 1·80–2·39; n=6259; CoE moderate) and second dose (2·06 [1·70–2·49]; n=6196; CoE moderate; figure 3; table). Observational data are provided in appendix 1 (pp 43–44).55 The risk for solicited systemic events was assessed in two phase 2/3 RCTs24, 25 and was slightly more frequent in vaccinated children than in unvaccinated children after the first dose (1·09 [1·04–1·16]; n=6259; CoE moderate) and increased after the second dose (1·49 [1·34–1·65]; n=6196; CoE moderate; figure 3; table). Observational data are provided in appendix 1 (pp 44–46).55
The risk for unsolicited adverse events increased for two-dose-vaccinated children compared with in unvaccinated children (RR 1·21 [95% CI 1·07–1·38; two phase 2/3 RCTs, n=6270; CoE moderate; figure 3). Recorded events of observational studies are provided in appendix 1 (pp 46–47).55
Regarding subgroup and sensitivity analyses, despite an increase in vaccine effectiveness with every additional vaccine dose, no relevant or consistent differences were observed between subgroups, although formal statistical analysis was not feasible (appendix 1 pp 52–54). Results remained robust in sensitivity analyses (appendix 1 pp 52–54).
Data on additional outcomes—ie, vaccine effectiveness against ICU admission due to COVID-19 and immunogenicity outcomes—are provided in appendix 1 (pp 48–51).
Discussion
In this systematic review on safety and effectiveness of EMA-approved COVID-19 vaccines in children aged 5–11-years, we found that a primary vaccination series with the mRNA-based vaccines (BNT162b2 and mRNA-1273) showed a high efficacy against SARS-CoV-2 infections and symptomatic COVID-19 in pivotal studies, which were done during a pre-omicron period, and not while the omicron variant was globally circulating. However, we identified a substantially lower vaccine effectiveness in real-life observations as reported in NRSIs, substantial heterogeneity in relative effect measures that could not be explained by potential effect modifiers (eg, type of vaccine, location, or baseline immunity), and further a rapidly waning vaccine-induced immunity from early follow-up data.40 Vaccine effectiveness against omicron infections and symptomatic COVID-19 could be increased through booster vaccination.32
The start of population-based vaccination coincided with the emergence of omicron, although both phase 2/3 RCTs were done while other variants of SARS-CoV-2 were circulating. Because a decreased effectiveness of COVID-19 vaccines against omicron was also observed in older age groups (≥12 years) since the shift from delta to omicron predominance,57, 58 differences in vaccine efficacy and effectiveness are most likely to be unrelated to the underlying study designs. Investigators of primary studies raised the concern that the waning effect might be enhanced through the lower vaccine dose (10 μg for age 5–11 years and 30 μg for age ≥12 years).40, 59 This waning was shown40 in a year-by-year age comparison with adolescents (aged 12–17 years), who received BNT162b 30 μg. Estimates of vaccine effectiveness against omicron in children aged 5–11 years were lower than those for children and adolescents aged 12–17 years, with a considerable difference between 11-year-old and 12-year-old children, who have similar physiology, but received different dosages (vaccine effectiveness in 11-year-olds was 11% [95% CI –3 to 23] and vaccine effectiveness in 12-year-olds was 67% [62 to 71]).40 This difference was even more profound over time, when vaccine effectiveness decreased from 65% (62 to 68) to below 0% for 5–11-year-olds at 35 days or more after a second dose, whereas vaccine effectiveness for 12–17-year-olds decreased from 76% (71 to 81) to 49% (34 to 60) in the same observation time.
Despite the initially low protection against omicron infections and symptomatic COVID-19, we identified considerable two-dose vaccine effectiveness against COVID-19-associated hospitalisations with moderate certainty in the evidence. A systematic review comparing vaccine effectiveness in children and adolescents showed no differences between age groups against COVID-19-related hospitalisations during the omicron period.59 Study data further suggest that COVID-19 vaccination protects well against MIS-C.54 However, because of substantial study limitations and the small sample size, we have little confidence that the observed effect is generalisable to all children. Although SARS-CoV-2 incidence rates were strongly increasing with the emergence of omicron, surveillance data suggest a decrease in MIS-C cases.60
Both mRNA vaccines frequently caused local or systemic reactions. Reactions were mostly mild and resolved within a few days. On the basis of RCT and observational data, we found no increased risk of serious adverse events. This finding was further supported through surveillance data showing a low incidence rate (ranging approximately 0·7–1·8 serious adverse events per 100 000 vaccinations; unpublished).61, 62 Similarly, no events of myocarditis were reported in RCTs, and single events with an estimated risk of 0·13–1·04 myocarditis cases per 100 000 vaccinations were reported in international vaccine safety surveillance systems.56 Thus, the risk of myocarditis appears substantially lower in children when compared with that of adolescents and young adults (22·15 myocarditis events per 100 000 vaccinations).63, 64 No new safety signals were identified in NRSIs.
Because RCTs are considered the most reliable evidence to assess efficacy of interventions,65 a key limitation of this systematic review was the limited applicability of identified RCT evidence to the current epidemiology. Also, despite large evidence base on initial vaccine effectiveness in children aged 5–11 years, follow-up time was short. Data for subpopulations (eg, children with immunocompromising conditions) were scarce and subgroup analyses were not feasible or not meaningful because many subgroup factors overlap between the included studies, increasing the risk for misleading causal interpretations when doing multiple subgroup analyses. Statistical pooling of immunogenicity data was not feasible or reasonable because of underlying heterogeneity and partially overlapping study populations.
Because the history of previous infections of included children was rarely reported and data analyses of remaining studies primarily included children who did not have a previous known SARS-CoV-2 infection, it remains unclear whether results are generalisable to children with previous infections. Given that the high seroprevalence in this age group,66 the additional benefit of vaccination in children aged 5–11-years cannot be reliably assessed with the underlying data of this systematic review. However, investigations of older age groups (≥16 years) suggest a preferable protective effect for individuals being both previously infected and vaccinated,67, 68 which was also suggested by data provided from Khan and colleagues32 for children aged 5–11 years.
A further methodological limitation of our review was the high proportion of non-peer reviewed studies (18 [35%] of 51 included studies). To address this limitation, we rigorously assessed risk of bias, and subsequently excluded NRSIs with a critical risk of bias from meta-analysis as suggested by ROBINS-I.15 However, with often only one remaining peer-reviewed study per outcome, planned sensitivity analyses were not meaningful. Nevertheless, whether peer-review affects the robustness of our findings is unclear.
Finally, the effect estimates underlying the calculated vaccine effectiveness varied across studies. Although vaccine efficacy from RCTs24, 25 was based on incidence rate ratios, considering person-years to address surveillance time, vaccine effectiveness from NRSIs was calculated on the basis of incidence rate ratios,29, 30, 40, 42, 49, 50 hazard ratios,43, 44, 47 RRs,45 or ORs.27, 28, 32, 46, 48, 51, 52, 53, 54 However, no outliers were identified in meta-analyses. Therefore, the calculation of vaccine effectiveness estimates are unlikely to have had a substantial effect on the overall effect.
In conclusion, a primary vaccination series with BNT162b2 and mRNA-1273 was probably highly effective in preventing SARS-CoV-2 infections and symptomatic COVID-19 in children aged 5–11 years before the emergence of omicron. Evidence of low certainty suggests that these vaccines are less effective against omicron infections, but still protect well against hospitalisations due to COVID-19. Little to no evidence exists on the risk of MIS-C or post-COVID-19 condition following SARS-CoV-2 infection in vaccinated children. Booster vaccination probably increases effectiveness, also against omicron infections. Evidence of moderate certainty suggests that vaccines are reactogenic but probably safe in the investigated age group. The risk of serious adverse events and myocarditis is uncertain, but observed event rates were low, and even lower after booster vaccination.
Continuous evaluation of the evidence is needed to assess the changing epidemiological landscape and to provide a solid base for medical guidelines and clinical decision making. Findings of this systematic review can serve as a basis for public health policy and individual decision making on COVID-19 vaccination in 5–11-year-olds.
For Covidence see https://www.covidence.org
For COVID-19 cases see https://covid19.who.int/
Data sharing
All data presented in this systematic review were obtained from published studies, which are cited in the references. Data extraction forms are available upon request from the corresponding author.
Declaration of interests
WS was employed at Roche from April, 2020, to June, 2021. Roche are not involved and have no influence on this project. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Marina Treskova and Guido Schwarzer, who provided us with their statistical expertise. This project was funded by the Federal Joint Committee (Gemeinsamer Bundesausschuss) in Germany (G-BA, 01VSF20019).
Contributors
VP, WS, JK, SV-B, JM, OW, and TH conceptualised the study. VP, WS, JJM, and TH designed the methodology. KK, KG, and VP did the literature search. VP, WS, and IT did the data extraction, risk of bias assessments, and GRADE assessments. WS and MT analysed and visualised the data. CB, OW, JJM, and TH contributed to the funding acquisition. JJM and TH supervised the study. VP, WS, IT, JJM, and TH validated the study data. WS, VL, and PK contributed to project administration. VP and WS wrote the original draft. All authors reviewed and edited the manuscript.
Supplementary Materials
References
- 1.European Medicines Agency EMA recommends first COVID-19 vaccine for authorisation in the EU. 2020. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
- 2.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert Koch Institut Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19), 12.01.2023—Aktualisierter Stand für Deutschland. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenberichte_Tab.html
- 4.Naeimi R, Sepidarkish M, Mollalo A, et al. SARS-CoV-2 seroprevalence in children worldwide: a systematic review and meta-analysis. EClinicalMedicine. 2023;56 doi: 10.1016/j.eclinm.2022.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statista Anzahl der Kinder und Jugendlichen (0- bis 17-Jährige) weltweit nach Altersgruppen im Jahr 2022. https://de.statista.com/statistik/daten/studie/1020443/umfrage/anzahl-der-kinder-und-jugendlichen-weltweit-nach-altersgruppen/
- 6.European Medicines Agency Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11. 2021. https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11
- 7.Martin B, DeWitt PE, Russell S, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID Cohort Collaborative. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.43151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimarães D, Pissarra R, Reis-Melo A, Guimarães H. Multisystem inflammatory syndrome in children (MISC): a systematic review. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14450. [DOI] [PubMed] [Google Scholar]
- 9.Butt AA, Dargham SR, Loka S, et al. Coronavirus disease 2019 disease severity in children infected with the omicron variant. Clin Infect Dis. 2022;75:e361–e367. doi: 10.1093/cid/ciac275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsche Gesellschaft für Pädiatrische Infektiologie e. V. PIMS-Survey-Update: 2022, Kalenderwoche 30. 2022. https://dgpi.de/pims-survey-update/
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90. doi: 10.1016/j.jclinepi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 13.WHO . World Health Organization; Oct 6, 2021. A clinical case definition of post COVID-19 condition by a Delphi consensus.https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Totten AM, Cheney TP, O'Neil ME, et al. Agency for Healthcare Research and Quality (US); Rockville, MD: 2018. Physiologic Predictors of Severe Injury: Systematic Review. [PubMed] [Google Scholar]
- 17.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. 2013. https://www.guidelinedevelopment.org/handbook
- 18.Schünemann HJ, Cuello C, Akl EA, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019;111:105–114. doi: 10.1016/j.jclinepi.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 20.Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20:1771–1782. doi: 10.1002/sim.791. [DOI] [PubMed] [Google Scholar]
- 21.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 22.Jackson D, Law M, Rücker G, Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med. 2017;36:3923–3934. doi: 10.1002/sim.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creech CB, Anderson E, Berthaud V, et al. Evaluation of mRNA-1273 COVID-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386:2011–2023. doi: 10.1056/NEJMoa2203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartsch YC, St Denis KJ, Kaplonek P, et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abn9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattiuzzi C, Lippi G. Real-world effectiveness of COVID-19 vaccination among children in Italy. Int J Infect Dis. 2022;122:70–71. doi: 10.1016/j.ijid.2022.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons AE, Amoako A, Grima A, Murison K, Tuite A, Fisman D. Vaccine effectiveness against hospitalization among adolescent and pediatric SARS-CoV-2 cases in Ontario, Canada. medRxiv. 2022 doi: 10.1101/2022.03.24.22272919. published online Sept 2. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung D, Rosa Duque JS, Yip KM, So HK, Wong WHS, Lau YL. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during Omicron BA.2 wave in Hong Kong. Commun Med. 2023;3:3. doi: 10.1038/s43856-022-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosa Duque JS, Leung D, Yip KM, et al. Effectiveness of BNT162b2 and CoronaVac against pediatric COVID-19-associated hospitalization and moderate-to-severe disease. medRxiv. 2022 doi: 10.1101/2022.09.09.22279426. published online Sept 9. (preprint) [DOI] [Google Scholar]
- 31.Wood N, Lopez LK, Glover C, et al. Active safety surveillance of COVID-19 mRNA vaccines in children aged 5-15 years in Australia. medRxiv. 2022 doi: 10.1101/2022.07.19.22277827. published online July 22. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan FL, Nguyen JL, Singh TG, et al. Estimated BNT162b2 vaccine effectiveness against infection with delta and omicron variants among US children 5 to 11 years of age. JAMA Network Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.46915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hause AM, Baggs J, Marquez P, et al. Safety monitoring of Pfizer-BioNTech COVID-19 vaccine booster doses among children aged 5-11 years—United States, May 17–July 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1047–1051. doi: 10.15585/mmwr.mm7133a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung D, Chan EY-h, Mu X, et al. Humoral and cellular immunogenicity and safety of 3 doses of CoronaVac and BNT162b2 in young children and adolescents with kidney diseases. medRxiv. 2022 doi: 10.1101/2022.09.14.22279916. published online Sept 15. (preprint) [DOI] [Google Scholar]
- 35.Straus W, Urdaneta V, Esposito DB, et al. Analysis of myocarditis among 252 million mRNA-1273 recipients worldwide. Clin Infect Dis. 2023;76:e544–e552. doi: 10.1093/cid/ciac446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suntronwong N, Vichaiwattana P, Klinfueng S, et al. SARS-CoV-2 infection- induced seroprevalence among children and associated risk factors during pre- and omicron-dominant wave, from January 2021 through November 2022, Thailand: longitudinal study. medRxiv. 2022 doi: 10.1101/2022.12.01.22283006. published online Dec 5. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastl AJ, Weaver KN, Zhang X, et al. Humoral immune response and safety of SARS-CoV-2 vaccination in pediatric inflammatory bowel disease. Am J Gastroenterol. 2023;118:129–137. doi: 10.14309/ajg.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 38.Hu M, Wong HL, Feng Y, et al. Results of safety monitoring of BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine in US children aged 5–17 years. medRxiv. 2022 doi: 10.1101/2022.10.28.22281532. published online Oct 30. (preprint) [DOI] [Google Scholar]
- 39.Hause AM, Marquez P, Zhang B, et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among children aged 5–11 years—United States, October 12–January 1, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:39–43. doi: 10.15585/mmwr.mm7202a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5-11 and 12-17 years in New York after the Emergence of the Omicron Variant. medRxiv. 2022 doi: 10.1101/2022.02.25.22271454. published online Feb 28. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi DS, Whitaker M, Marks KJ, et al. Hospitalizations of children aged 5–11 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:574–581. doi: 10.15585/mmwr.mm7116e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amir O, Goldberg Y, Mandel M, et al. Initial protection against SARS-CoV-2 omicron lineage infection in children and adolescents by BNT162b2 in Israel: an observational study. Lancet Infect Dis. 2023;23:67–73. doi: 10.1016/S1473-3099(22)00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chemaitelly H, AlMukdad S, Ayoub HH, et al. COVID-19 vaccine protection among children and adolescents in Qatar. N Engl J Med. 2022;387:1865–1876. doi: 10.1056/NEJMoa2210058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cocchio S, Zabeo F, Tremolada G, et al. COVID-19 vaccine effectiveness against omicron variant among underage subjects: the Veneto region's experience. Vaccines. 2022;10 doi: 10.3390/vaccines10081362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen-Stavi CJ, Magen O, Barda N, et al. BNT162b2 Vaccine effectiveness against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–236. doi: 10.1056/NEJMoa2205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years—PROTECT Cohort, July 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:422–428. doi: 10.15585/mmwr.mm7111e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang EJ, Choe YJ, Kim RK, Park Y-J. BNT162b2 vaccine effectiveness against the SARS-CoV-2 omicron variant in children aged 5–11 years. JAMA Pediatr. 2023;177:319–320. doi: 10.1001/jamapediatrics.2022.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327:2210–2219. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacco C, Del Manso M, Mateo-Urdiales A, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January-April, 2022. Lancet. 2022;400:97–103. doi: 10.1016/S0140-6736(22)01185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan S, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years. SSRN. 2022 doi: 10.2139/ssrn.4052133. published online March 28. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piché-Renaud PP, Swayze S, Buchan S, et al. Vaccine effectiveness of BNT162b2 against omicron in children aged 5–11 years: a test-negative design. SSRN. 2022 doi: 10.2139/ssrn.4176388. published online Aug 1. (preprint) [DOI] [Google Scholar]
- 52.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years—VISION Network, 10 States, April 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. doi: 10.15585/mmwr.mm7109e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zambrano LD, Newhams MM, Olson SM, et al. BNT162b2 mRNA vaccination against COVID-19 is associated with decreased likelihood of multisystem inflammatory syndrome in US children Ages 5–18 Years. Clin Infect Dis. 2022;76:e90–100. doi: 10.1093/cid/ciac637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hause AM, Shay DK, Klein NP, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics. 2022;150 doi: 10.1542/peds.2022-057313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nygaard U, Holm M, Dungu KHS, et al. Risk of myopericarditis after COVID-19 vaccination in Danish children aged 5 to 11 years. Pediatrics. 2022;150 doi: 10.1542/peds.2022-057508. [DOI] [PubMed] [Google Scholar]
- 57.Külper-Schiek W, Piechotta V, Pilic A, et al. Facing the omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.940562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Liang H, Ding X, Cao Y, Yang D, Duan Y. Effectiveness of COVID-19 vaccine in children and adolescents with the Omicron variant: a systematic review and meta-analysis. J Infect. 2023;86:e64–e66. doi: 10.1016/j.jinf.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen JM, Carter MJ, Cheung CR, Ladhani S. Lower risk of multisystem inflammatory syndrome in children with the delta and omicron variants of severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2023;76:e518–e521. doi: 10.1093/cid/ciac553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paul-Ehrlich-Institut Sicherheitsbericht. Bundesinstitut für Impfstoffe und biomedizinische Arzneimittel. 2022. https://www.pei.de/DE/newsroom/dossier/coronavirus/arzneimittelsicherheit.html
- 62.Public Health Agency of Canada Canadian COVID-19 vaccination safety report. February 11, 2022. https://health-infobase.canada.ca/covid-19/vaccine-safety/
- 63.Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022;176:612–614. doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pillay J, Gaudet L, Wingert A, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following COVID-19 vaccination: living evidence syntheses and review. BMJ. 2022;378 doi: 10.1136/bmj-2021-069445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005;90:840–844. doi: 10.1136/adc.2004.058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maier BF, Rose AH, Burdinski A, et al. Estimating the distribution of COVID-19-susceptible, -recovered, and -vaccinated individuals in Germany up to April 2022. medRxiv. 2022 doi: 10.1101/2022.04.19.22274030. published online April 23. (preprint) [DOI] [Google Scholar]
- 67.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this systematic review were obtained from published studies, which are cited in the references. Data extraction forms are available upon request from the corresponding author.