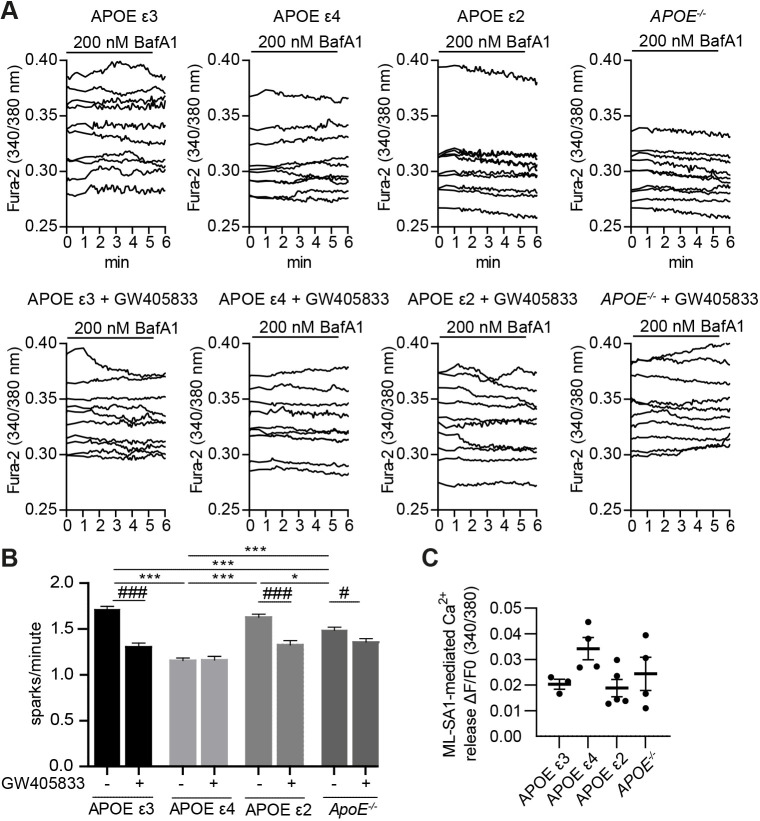
Fig. 4.
Decreased TRPML1-mediated lysosomal Ca2+ release in a neuronal LOAD iPSC model. (A,B) APOE ε3, APOE ε4, APOE ε2 or APOE−/− iPSC neurons were loaded with Fura2-AM and treated with the IP3 receptor antagonist xestospongin C to block Ca2+ efflux from the ER, followed by BafA1 to mimic age-related mild deacidification. TRPML1 activity was assessed by counting spontaneous sparks of Ca2+ release during a 5-min period in the presence [APOE ε3 (n=11, 235 traces), APOE ε4 (n=12, 243 traces), APOE ε2 (n=10, 226 traces), APOE−/− (n=11, 257 traces)] or absence [APOE ϵ3 (n=6, 90 traces), APOE ϵ4 (n=6, 101 traces), APOE ϵ2 (n=6, 130 traces), APOE−/− (n=7, 163 traces)] of the TRPML1 inhibitor GW405833 (10 µM). The response of representative cells is depicted and expressed as the 340/380 nm ratio of Fura2-AM florescence. Representative traces (A) and quantification (B) of spark number/minute induced by BafA1. Significance levels were calculated between all samples without GW405833 to detect APOE isoform-specific alterations in TRPML1 response (*P<0.05; **P<0.01; ***P<0.001, one-way ANOVA followed by Bonferroni post-hoc test) and for each APOE isoform between GW405833-treated and untreated sample to assess TRPML1 contribution (#P<0.05; ###P<0.001, unpaired two-tailed Student's t-test). (C) Quantification of full physiological cellular Ca2+ release after addition of 10 µM ML-SA1 including, but not limited to lysosomal Ca2+ stores in n=3 biological replicates, n=1 technical replicate per APOE isoform. Data are expressed as mean±s.e.m.