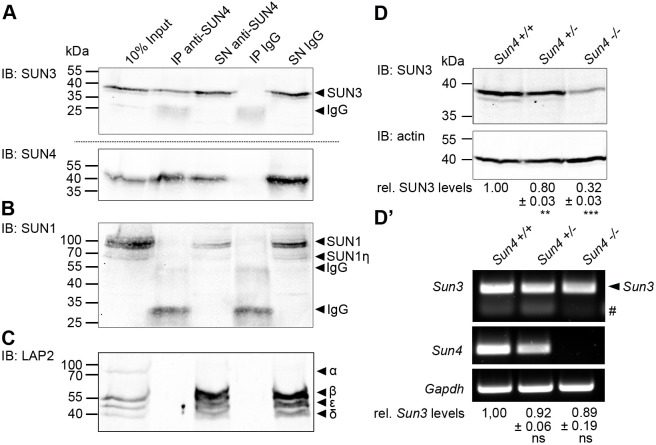
Fig. 6.
SUN4 forms heteromeric assemblies with SUN3 in mouse spermatids and SUN3 protein levels are decreased in SUN4 knockout tissue. (A–C) Western blots of rabbit anti-SUN4 immunoprecipitated membrane protein extracts from mouse testis cells. Non-specific rabbit IgGs were used as a negative control. Precipitates (IP) and supernatants containing unbound proteins (SN) were probed with (A) guinea pig anti-SUN3 and anti-SUN4, (B) anti-SUN1 and (C) mouse anti-LAP2 antibody (immunoblot, IB). Untreated testis cells served as input control. LAP2 isoforms were assigned according to Berger et al. (1996). Please note that LAP2α does not contain a TMD and thus can not be present in the SN. Amounts of loaded cell equivalents: 10% input, 1.5×106; IP, 1.5×107; SN, 1.5×107. Images in A–C are representative of at least two experimental repeats. (D) Immunoblot detection of SUN3 levels in Sun4+/+, Sun4+/− and Sun4−/− whole-testis lysates; actin protein levels served as loading control. Relative SUN3 protein expression levels normalized to actin (±s.d. from three independent experiments) are given below. (D′) Semi-quantitative RT-PCR analysis of Sun3 mRNA levels in Sun4+/+, Sun4+/− and Sun4−/− mouse testis cells. Sun4 and Gapdh mRNA levels were monitored as control. Relative Sun3 mRNA levels normalized to Gapdh (±s.d. from three independent experiments) are shown below. Hash symbol indicates background signals of the primers. Two-tailed one-sample t-tests were performed to valuate the significance of the differences between Sun3 mRNA and SUN3 protein levels in Sun4+/+, Sun4+/− and Sun4−/− tissues. ns, not significant (P>0.05); *P≤0.05; **P≤0.01; ***P≤0.001.