Fig. 1.
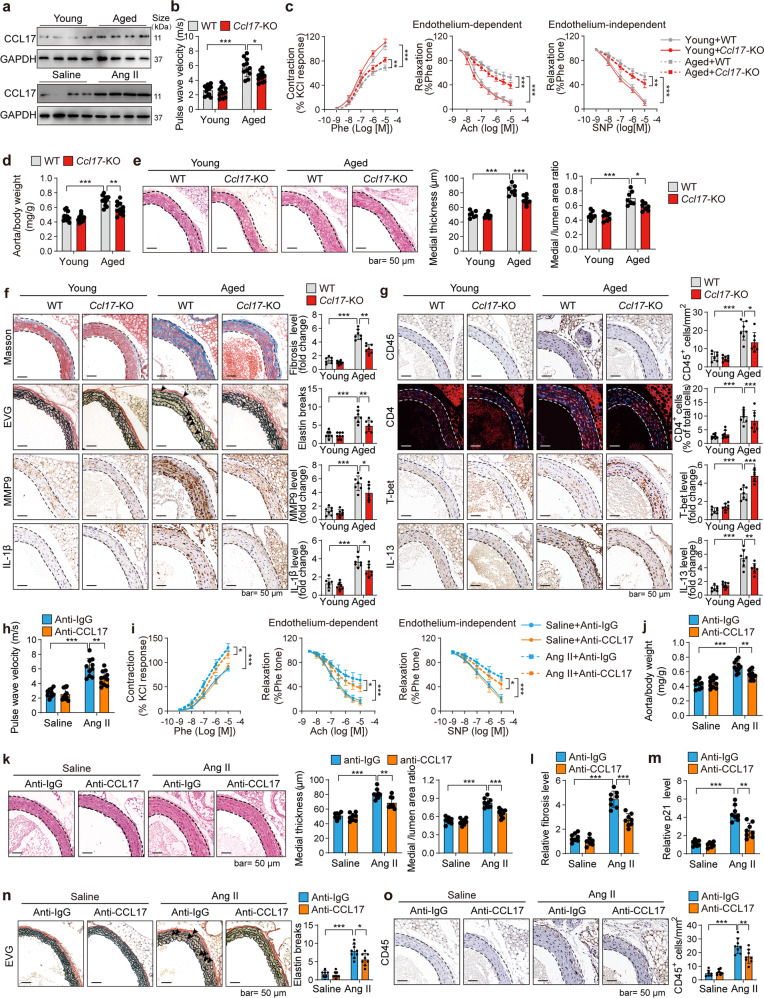
Knockout and inhibition of CCL17 inhibit aging or Ang II-induced vascular dysfunction. a CCL17 protein level was increased in the aortas from aged or angiotensin II (Ang II)-treated mice. For the aging model, the aortas of young (4-month) and aged (21-month) mice were analyzed. For Ang II-treated model, Ang II (1.3 mg/kg/day) was applied to challenge young wild-type (WT) C57BL/6 mice for 28 days to induce vascular remodeling, and then the aortas were analyzed. Ccl17 knockout inhibited aging-induced vascular dysfunction. The aortas of young (4-month) and aged (21-month) WT and Ccl17 knockout (Ccl17-KO) male C57BL/6 mice were analyzed. b Pulse wave velocity (PWV) measurement in young and aged mice revealed that Ccl17 knockout inhibited the aging-induced increase in arterial stiffness (n = 11, 12). c Ex vivo analysis of the vascular constriction-relaxation function of aortas. (left) the arterial vessel contractions mediated through phenylephrine; (middle) the endothelium-dependent relaxation responding to acetylcholine; (right) the endothelium-independent relaxation responding to sodium nitroprusside (n = 6). d Ccl17 knockout reduced the aorta-weight to the body-weight ratio in aged mice (n = 11, 12). e Ccl17 knockout reduced vascular remodeling in aged mice; hematoxylin-eosin (H&E) staining of the thoracic aortas was performed, and the quantitative results of the medial thickness as well as the media-area/vessel-lumen ratio are shown (n = 7, 8). f Ccl17 knockout reduced fibrosis (Masson staining), elastin fiber breakage (EVG staining), and expression of biomarkers of senescence-associated secretory phenotypes (immunohistochemical staining of MMP9 and IL-1β) in aged aortas. Representative images and quantitative results are shown (n = 7, 8). g Ccl17 knockout regulated Th1 (T-bet) and Th2 (IL-13) cells in aged aortas. Immunohistochemical staining was performed to analyze total immune cells (CD45), Th1 marker (T-bet), and Th2 marker (IL-13). Immunofluorescence staining was performed to analyze total CD4+ T cells. Representative images and quantitative results are shown (n = 7, 8). CCL17 neutralizing antibody inhibited Ang II-induced vascular dysfunction. Young (4-month) WT mice were challenged by Ang II (1.3 mg/kg/day) and treated with CCL17 neutralizing antibody (anti-CCL17, 100 μg per day) or control isotype antibody (anti-IgG, 100 μg per day) for four weeks. h PWV measurement revealed that the CCL17 antibody repressed Ang II-induced increase in arterial stiffness (n = 9–11). i Ex vivo analysis of the vascular constriction-relaxation function of aortas from mice. (left) the arterial vessel contractions mediated through phenylephrine; (middle) the endothelium-dependent relaxation responding to acetylcholine; (right) the endothelium-independent relaxation responding to sodium nitroprusside (n = 6). j CCL17 antibody decreased the aorta-weight/body-weight ratio in Ang II-treated mice (n = 9–11). k CCL17 antibody reduced remodeling of aortas in Ang II-challenged mice; H&E staining of the thoracic aortas was performed, and the quantitative results of medial thickness and the media-area/vessel-lumen ratio are shown (n = 7–9). l CCL17 antibody reduced vascular fibrosis (Masson staining) in Ang II-treated mice (n = 7, 8). m CCL17 antibody reduced the senescence marker p21 in aortas from Ang II-challenged mice (n = 7, 8). n EVG staining showing CCL17 antibody reduced breakage of elastin fiber in the aortas from Ang II-challenged mice (n = 7, 9). o CCL17 antibody reduced immune cell (CD45) infiltration in the aortas from Ang II-challenged mice (n = 7, 8). CCL17, C-C motif chemokine ligand 17; EVG, elastic van Gieson; IL-1β, Interleukin-1β; MMP9, matrix metalloproteinase-9. All the data are presented as mean ± SD. When the assumptions were satisfied, a two-way ANOVA test was applied with Bonferroni post-hoc test for comparison. Otherwise, we used Kruskal–Wallis test with Dunn’s post hoc test for comparison. *P < 0.05, **P < 0.01, ***P < 0.001