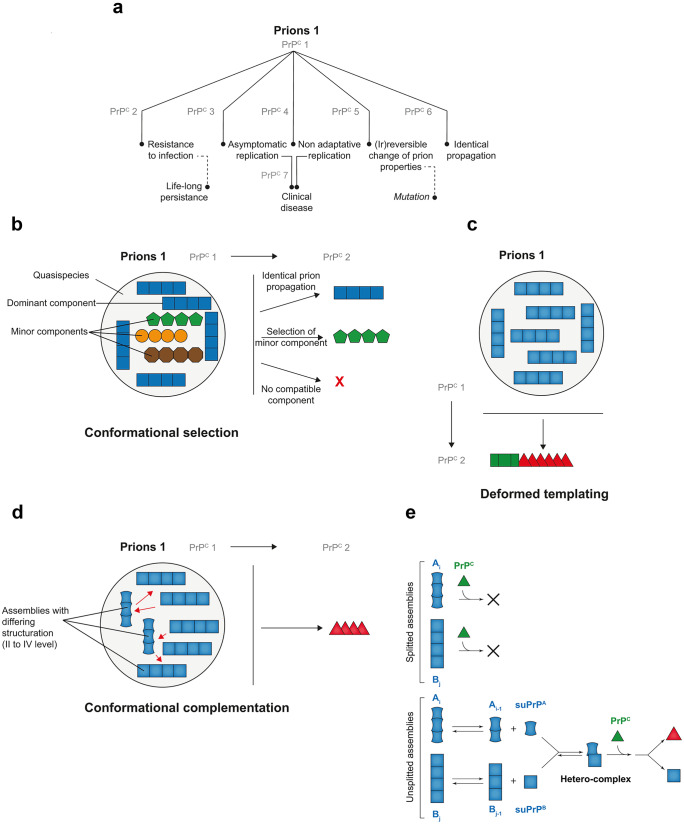
Fig. 4.
Molecular determinants of prion species barrier. a Main outcomes observed during experimental prion cross-species transmission (or transmission to heterologous PrPC as done by transgenic modeling). To be pointed out, cross-species transmission of a different prion from species/PrPC 1 may lead to drastically different outcomes. b Conformational selection model to explain prion cross-species transmission at the molecular level. In this model, prions would be composed of a cloud of subcomponents or substrains in varying proportions. The major component would be responsible for the strain phenotype in the parental host species. Other substrains would be co-propagated as minor components. On cross-species transmission, the optimized subcomponent, i.e., the component that lies within the portfolio of acceptable conformations in the new PrPC species, would be preferentially selected. The issue of the transmission will thus mostly depend on the presence of a compatible component and relative concentration. c In the deformed templating model, prion primary passage to species expressing heterologous PrPC would be inefficient, because of structural incompatibility between infecting PrPSc and PrPC. This would lead to generation of PrPSc with atypical conformation (green square) in a reduced number of asymptomatic animals. On subpassage, this conformation would slowly evolve toward an optimized conformation (red triangle), allowing adaptation. d–e In the conformational complementation model (d), prions would be composed of heterogeneous PrPSc assemblies with respect to secondary, tertiary, and quaternary structure. Interaction between these assemblies (red arrows) would allow crossing the species barrier. Mechanistically (e), the complex formed by the suPrPs from the different PrPSc assemblies (here Ai and Bj) would provide an interaction interface with heterologous PrPC that is absent in each assembly or each individual suPrP