Abstract
Prion diseases (PrD) or transmissible spongiform encephalopathies (TSE) are invariably fatal and pathogenic neurodegenerative disorders caused by the self-propagated misfolding of cellular prion protein (PrPC) to the neurotoxic pathogenic form (PrPTSE) via a yet undefined but profoundly complex mechanism. Despite several decades of research on PrD, the basic understanding of where and how PrPC is transformed to the misfolded, aggregation-prone and pathogenic PrPTSE remains elusive. The primary clinical hallmarks of PrD include vacuolation-associated spongiform changes and PrPTSE accumulation in neural tissue together with astrogliosis. The difficulty in unravelling the disease mechanisms has been related to the rare occurrence and long incubation period (over decades) followed by a very short clinical phase (few months). Additional challenge in unravelling the disease is implicated to the unique nature of the agent, its complexity and strain diversity, resulting in the heterogeneity of the clinical manifestations and potentially diverse disease mechanisms. Recent advances in tissue isolation and processing techniques have identified novel means of intercellular communication through extracellular vesicles (EVs) that contribute to PrPTSE transmission in PrD. This review will comprehensively discuss PrPTSE transmission and neurotoxicity, focusing on the role of EVs in disease progression, biomarker discovery and potential therapeutic agents for the treatment of PrD.
Keywords: Prion disease, Extracellular vesicle, Neurotoxicity
Introduction
Prion diseases (PrD) or transmissible spongiform encephalopathies (TSE) are progressively rapid and fatal neurodegenerative diseases (NDs) with a defining hallmark of vacuolation in the brain tissue (Besnoit and Morel 1898). Similar to other NDs with specific protein misfolding–related neurodegeneration, PrD consists of a pathogenic form (PrPTSE) of normal cellular prion protein (PrPC) (Prusiner 1998). However, unlike other NDs, the unique interspecies transmissibility and pathogenic nature of PrD make PrD more hazardous (Beck et al. 1969; Gajdusek et al. 1966; Gibbs et al. 1968). There are various forms of animal and human prion diseases, with one of the highly studied animal PrD being bovine spongiform encephalopathy (BSE; also known as ‘mad cow disease’) in cattle which was discovered in the UK for the first time in 1984–1985 (Wells et al. 1987). Soon after the UK BSE outbreak, a new form of human prion disease emerged with distinct pathophysiology termed variant CJD (vCJD) (Will et al. 1996). Thus, the spread of vCJD has been associated with the consumption of BSE-infected meat products. Other animal prion diseases include transmissible mink encephalopathy (TME) in mink, chronic wasting disease (CWD) in cervids, feline spongiform encephalopathy in domestic cats and exotic ungulate encephalopathy (EUE) in Nyala, greater kudu and oryx. A new animal prion disease in the dromedary camel of Algeria has been detected with different biochemical properties of PK-resistant prion protein to that of BSE and scrapie (Babelhadj et al. 2018).
Human PrD are differentiated into three types based on aetiologies which include genetic, sporadic and acquired. PrD, which fall under the genetic aetiologies due to point mutations or octapeptide repeat insertions in the PRNP gene, includes familial CJD (fCJD), Gerstmann–Stäussler–Scheinker (GSS) and fatal familial insomnia (FFI) while sporadic CJD (sCJD) presents with disease aetiology from unknown origins. PrD with acquired aetiologies from exposure to pathogenic prions includes iatrogenic CJD (iCJD), vCJD, and kuru. To date, various human-to-human iCJD transmissions occurred due to incomplete decontamination of surgical equipment, corneal grafting, dura mater grafting, cadaveric pituitary-derived growth hormone or gonadotrophin, and blood transfusions (Brown et al. 2006). The approximate prevalence of human PrD equates to 85–90% for sCJD cases, 10% for fCJD cases and less than 2–5% for acquired CJD (Chen and Dong 2016). Although the acquired CJD cases are of lower prevalence, the experimental disease transmissibility rate from humans to primates was highest in acquired CJD compared to sCJD and fCJD (Brown et al. 1994).
Protein-only hypothesis of prion replication
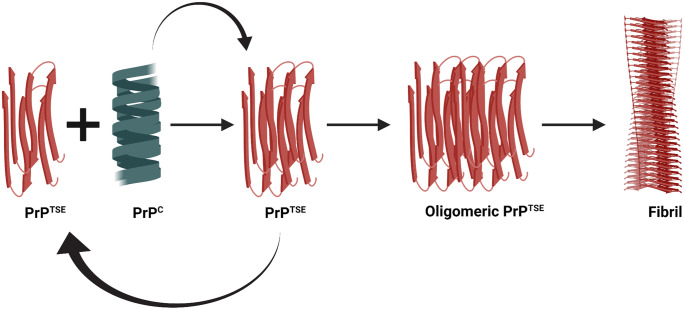
Extensive purification of the scrapie prion protein with subsequent sodium dodecyl sulphate (SDS) denaturation, proteinase K (PK) treatment and high-performance liquid chromatography (HPLC) purification yielded the significant component of a single protein ranging 27–30 kDa in size (PrP 27–30) (Prusiner et al. 1984). This purified PrP27–30 amino acid sequencing and the direct amino acid sequencing of the purified scrapie rods produced identical 15 N-terminal amino acid residues indicating that scrapie rods are a polymer of identical proteins. Next, the same gene encoded the healthy and disease-associated forms of prion protein in both human and mouse (Basler et al. 1986, Oesch et al. 1985). Moreover, a single uninterrupted coding gene for the prion protein suggests that the difference in healthy and scrapie associated prion protein could arise from post-translational modifications, sequence variations or conformational changes, not due to an alternative splicing mechanism (Basler et al. 1986). Subsequent experiments transferring mouse scrapie prions into mice devoid of prion protein demonstrated that these did not develop the disease and showed no behavioural changes (Bueler et al. 1993). All these attempts to determine the infectious nature of PrD led to the formation of the protein-only hypothesis, which considers the PrPTSE isoform of the normal PrPC protein as the sole causative agent of disease transmission (Prusiner 1998) (Fig. 1). The generation of the PrPTSE isoform is either due to a gene mutation or via exogenous PrPTSE exposure, and PrPTSE would subsequently seed propagating the conversion of PrPC into PrPTSE in an autocatalytic fashion.
Fig. 1.
Schematic diagram illustrating the protein-only hypothesis. Here, the pathogenic form of prion protein (PrPTSE) is the sole causative agent of the disease which acts as a seed in the cyclic conversion of normal cellular prion (PrPC) into the misfolded pathogenic isoform (PrPTSE) in an autocatalytic fashion. These newly formed PrPTSE further acts as a seed to this conversion mechanism to form PrPTSE oligomers and fibrils. This figure was generated using Biorender
PrPC structure, biogenesis and physiological function
PrPC has high endogenous expression in neurons and is encoded by only one exon out of three exons in the PrPC gene at chromosome 20 (Basler et al. 1986). The 253 amino acid sequence of prion protein contains a signal peptide in the first 22 amino acids, which is cleaved once it reaches the ER. PrPC undergoes various post-translational modifications, which add a glycosylphosphatidylinositol (GPI) anchor at the C terminus (residue 230) and two N-linked glycans at residues 181 and 197. Nuclear magnetic resonance (NMR) examination of PrPC structure revealed an intrinsically disordered N-terminal tail (residues 23–128), three α-helical regions (two of them linked by a disulphide bridge) and a short anti-parallel β-sheet (Riek et al. 1997). The N-terminal region contains four identical, highly conserved octapeptide repeats (residues 51–91) (Kim et al. 2008). In the mature form, PrPC is translocated to the outer leaflet of the plasma membrane in lipid raft regions and a significant portion to the non-raft regions (Sarnataro et al. 2004; Sunyach et al. 2003). The cell surface PrPC is rapidly endocytosed and recycled back via coated pits (Shyng et al. 1994; Sunyach et al. 2003). In the cytosol, most PrPC is found in the multivesicular bodies (MVBs), where it is packaged in extracellular vesicles (EVs; endosomal derived exosomes) and released into the extracellular environment (Guo et al. 2015; Mironov et al. 2003; Yim et al. 2015).
Although early experiments showed PrPC knock-out mice exhibited no significant changes in behaviour and phenotype, a growing number of cell culture–based models attribute multimeric functions to PrPC (Büeler et al. 1992). Using a mass spectrometry approach, Zafar and colleagues (2011) showed many PrPC binding partners with primary predicted functions, including cell growth, signal transduction, cellular metabolism and stress pathways (Zafar et al. 2011). In addition, PrPC maintains neuronal development (Steele et al. 2006), neurite outgrowth (Santuccione et al. 2005), synapse health (Laurén et al. 2009; Šišková et al. 2013), myelin maintenance (Bremer et al. 2010; Küffer et al. 2016), cellular metal ion homeostasis (Pauly and Harris 1998; Thompsett et al. 2005; Watt et al. 2012), circadian rhythm regulation (Huber et al. 2002; Tobler et al. 1996), protection from stress (Rachidi et al. 2003; Zanata et al. 2002) and neural cell adhesion molecule 1 (NCAM 1) deregulations (Mehrabian et al. 2016; Schmitt-Ulms et al. 2001). Most recently, the N-terminal copper-binding site of PrPC was demonstrated to be essential for neuronal protection and neuritogenesis (Nguyen et al. 2019). In addition, to overcome the issue with genetic artefacts associated to lack of proper PrPC knockout models in several of the PrPC functions indicated above, a more physiologically relevant co-isogenic Prnp0/0 mouse model (PrnpZH3/ZH3) was developed (Matamoros-Angles et al. 2022; Nuvolone et al. 2016). The co-isogenic PrPC knockout model studies demonstrated a vital role in peripheral myelin maintenance, neural network formations, synaptic regulations and cognitive abilities (Matamoros-Angles et al. 2022; Nuvolone et al. 2016). Thus, multiple functions of PrPC suggest that the abnormal conformation due to conversion into PrPTSE generates potential disturbances in various biological processes leading to pathogenesis in PrD.
Structure of PrPTSE
To elucidate the nature of PrPTSE and its autocatalytic propagation, it is essential to understand the structure of PrPTSE. Unlike PrPC, the aggregated, insoluble and variably post-translationally modified nature of PrPTSE has hampered high-resolution structural identification. Early biophysical analysis demonstrated that PrPTSE possesses an increased β-sheet content and reduced α-helix content to PrPC (Caughey et al. 1991). Further advancement on the structural analysis of PrPTSE includes electron microscopy of N-terminally truncated isomorphous 2D scrapie prion crystals (Wille et al. 2002) and the refinement in modelling of this structure (Govaerts et al. 2004). Together, these studies predicted that the amyloids contain the trimeric parallel left-handed β-sheet structure of PrPTSE. The previously predicted model incorporated the α-helix from the PrPC structure, which was ruled out with hydrogen–deuterium exchange-coupled mass spectrometry analysis of mammalian PrPTSE (Smirnovas et al. 2011). Although consistent with the previous electron crystallography study, the PrPTSE core consisted of β-sheet structure with short turns and/or loops.
Subsequent studies using transgenic mice expressing mammalian GPI-less PrPTSE exhibiting limited proteolysis with proteinase K coupled mass spectrometry predicted the four-rung β-solenoid (4RβS) model (Silva et al. 2015; Vazquez-Fernandez et al. 2012). The 4RβS model consists of proteinase K–sensitive, flexible loops or stretches that connect the highly compact β-strand solenoid core. This 4RβS core of PrPTSE originally came from the X-ray fibre diffraction experiment of infectious prions (Wille et al. 2009). Recent support of the predicted 4RβS model of PrPTSE came from the electron cryomicroscopy analysis of GPI-less amyloid fibril, demonstrating an average fibril repeating unit of 19.1 Å (Vazquez-Fernandez et al. 2016). Next, a comprehensive atomistic analysis using experimental and computational findings presented a more physically plausible model supporting 4RβS and with the stability equivalent to a naturally occurring β-solenoid protein (Spagnolli et al. 2019). The simulations performed in this study constructed an underlying mechanism of PrPTSE autocatalysis, which proposed a sequential addition of rungs where the C-terminal rung of the β-solenoid acts as a template in the conversion of PrPC to PrPTSE. Strengthening the 4RβS model of PrPTSE, isolation of infectious BSE prions for electron microscopy experiments and three-dimensional construction analysis showed rod-shaped fibrillar morphology with configuration aligning to that of the compact 4RβS model (Kamali-Jamil et al. 2021). While most amyloid studies support the four-rung β-solenoid model, other studies using solid-state NMR and side-directed spin labelling in recombinant amyloid fibrils demonstrated that PrPTSE fibrils exhibit parallel-in-register intermolecular β-sheet (PIRIBS)–based structure (Cobb et al. 2007; Groveman et al. 2014; Tycko et al. 2010). A recent study supporting PIRIBS model with near atomic resolutions from electron cryomicroscopy was obtained from fully infectious scrapie prion isolates (Kraus et al. 2021). To better solve the structure of PrPTSE, both models should be considered to incorporate PrPTSE complexity regarding different conformations and infectivity levels.
Prion strain variation and species barriers
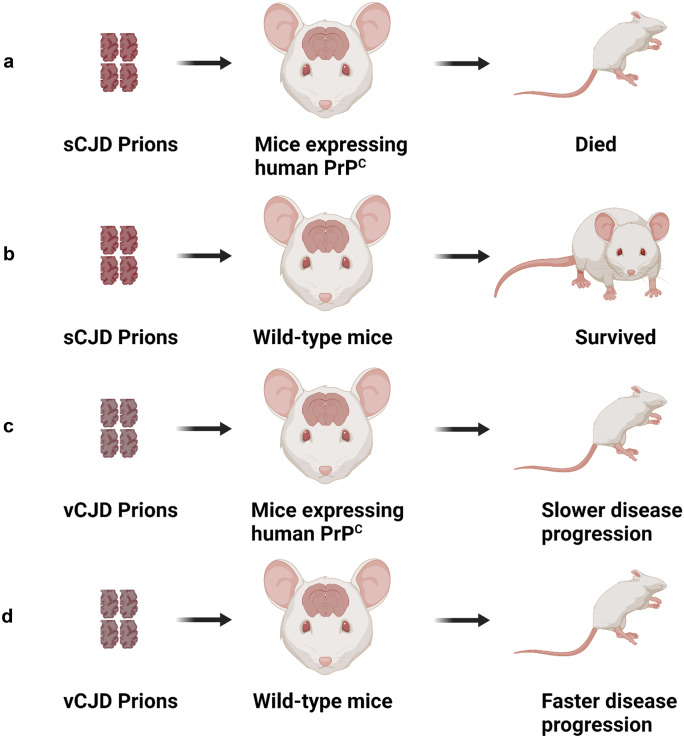
The numerous types of prion disease find their origins in different strains of the pathogenic prion protein that exhibit different biochemical profiles, incubation times and distribution of brain PrPTSE lesions. A new form of prion strain can be established when the PrPTSE from one animal species is transmitted and serially passaged to a different species (Kimberlin and Walker 1978). Primarily, strain tropism is shaped from the host PrPC gene sequence and other possible host environmental factors such as lipids, RNA, chaperones and glycosaminoglycans (Baron and Caughey 2003; Geoghegan et al. 2007). Prion disease transmission from one animal species to another can have a varying degree of species barrier based on the efficiency of disease propagation and onset of clinical symptoms, which has been linked to the prion strain differences. A classic example of this is the successful infection of transgenic mice expressing human prions but not the wild-type mice when infected with human sCJD prion seeds (Collinge et al. 1995).
Conversely, vCJD prions successfully infected mice PrPC more efficiently than transgenic mice expressing only human PrPC (Collinge et al. 1996) (Fig. 2). Later, it was discovered that transgenic mice expressing human PrPC with codon 129 methionine homozygosity were necessary for causing vCJD disease phenotype and circumventing the species barrier (Wadsworth et al. 2004). In vitro studies demonstrated that species barriers could be bypassed by single amino acid residue substitutions in a critical region of the PrPC sequence that removes the variations between two species (Jones and Surewicz 2005; Vanik et al. 2004). More importantly, another in vitro study demonstrated that the differences in secondary structures of PrPTSE fibrils result in a species barrier, with new strains bypassing the species barrier inheriting the secondary structure and morphology of the seed fibril (Jones and Surewicz 2005). This implies that the different conformations of PrPTSE are transmitted as different quaternary structures to form the basis of the species barrier. A novel study utilising asymmetric-flow field-flow fractionation (AF4) isolation of infectious prions from different prions strains for dynamic and multi-angle light scattering (DLS/MALS) analysis showed quaternary structure difference resulting in PrPTSE strain heterogeneity (Cortez et al. 2021).
Fig. 2.
Schematic diagram illustrating the classical model of species barrier associated strain differences. Only mice expressing human cellular prion protein (PrPC) succumb to disease, while wild-type mice remain resistant when injected with sporadic CJD (sCJD) prion strain. However, infection with a different prion strain of variant CJD (vCJD) is capable of bypassing the species barrier, and wild-type mice succumb to the disease. This figure was generated using Biorender
Strain-specific tropism produces various clinicopathological features and regional specific PrPTSE deposition in addition to variation in incubation time and disease duration. In terms of M1000 and MU02 prion strains, which are mouse adapted human prion strains of familial and sporadic origins, respectively, both the thalamus and hippocampus demonstrated spongiform changes. At the same time, PrPTSE plaques were present only in thalamus for MU02 (Lawson et al. 2008). The molecular basis of strain tropism-related unique disease variables has been associated with the different conformations of PrPTSE. Moreover, differences in the clinicopathological features between two familial forms of PrD, which included widespread spongiform degeneration in fCJD and selective thalamic nuclei atrophy FFI, resulted from different PrPTSE isoforms determined by a codon 129 methionine/valine (M/V) polymorphism (Goldfarb et al. 1992). Similarly, in terms of distinct pathology of sCJD, the associated variables were demonstrated to be a M/V polymorphism at codon 129 of PRNP as well as different PK cleavage profiles of protease-resistant core fragments (PrPres) (Hill et al. 2003; Kobayashi et al. 2007; Parchi et al. 2009). Based on the migration of the unglycosylated PK resistant (PrPres) fragment on SDS-PAGE gels, three different PrPres subtypes have been identified, which include type 1 (21 kDa), type 2 (19 kDa) and type intermediate (i;20 kDa) (Parchi et al. 2009). Recently, a study demonstrated the involvement of the PrPC GPI anchor in determining the species barrier by detecting increased infectivity of GPI-deficient mouse scrapie PrPTSE to the tg44 mice overexpressing human PrPC (Race et al. 2015). Although structural differences of PrPTSE have been proven to be the primary source of the species barrier, other host environmental factors should be considered for having a crucial role in transferring infectivity.
Protein misfolding cyclic amplification (PMCA) is a technique that converts normal brain homogenate PrPC substrate into pathogenic PrPTSE form using multiple sonication/incubations in the presence of a PrPTSE seed. It is concerning that strain-specific interspecies barrier-crossover was detected with PMCA when stabilised or adapted cervid PrPTSE seed successfully converted transgenic mice overexpressing human PrPC substrate (Barria et al. 2011). Successful adaptation of this natural source–extracted cervid PrPTSE seed was performed with one or two passages in a PMCA experiment using mice overexpressing cervid PrPC as a substrate and in an in vivo experiment using mice overexpressing cervid PrPC. In addition, other studies involving strain adaptation using in vivo models and PMCA experiments demonstrated that the adapted strain had increased infectivity in subsequent passaging to the adaptation species (Barria et al. 2011; Chianini et al. 2012). However, one recent study demonstrated the existence of a non-adaptive form of prion replication using in vivo models and PMCA, with transgenic mice expressing horse and deer PrPC retaining strain properties of source PrPTSE (Bian et al. 2017). Overall, it is essential to note that the interspecies barrier potentially depends both on the strain types and the degree of the strain adaptation.
PrPTSE and neurotoxicity
Even though neuronal loss and PrPTSE deposition are the characteristic features of prion-infected brains, the mechanism of neurotoxicity has remained elusive. However, several studies have investigated the synergistic effect generated by the interaction of PrPC and PrPTSE, providing some explanation on the neurotoxic effects of PrPTSE. Grafting neural tissue overexpressing PrPC into PrPC null mice brains and intracerebrally injecting scrapie prions produced severe histopathological changes exclusively in the grafted tissue (Brandner et al. 1996). Significant amounts of PrPTSE were further detected in the surrounding brain regions devoid of PrPC which failed to elicit histopathological changes even after 16 months. The discovery that PrPC expression in the host species was directly proportional to the clinical onset of the disease suggested that the PrPC/PrPTSE interaction catalyses the production of neurotoxic species (Sandberg et al. 2011). Moreover, a growing body of evidence suggests cell membrane PrPC elicits downstream neurotoxicity following its interaction with PrPTSE (Altmeppen et al. 2015; Herrmann et al. 2015; Resenberger et al. 2011a).
It has been demonstrated that the small oligomeric unit of PrP with a mass equivalent to 14–28 PrP units exhibits more infectivity than higher fibrillar amyloids (Silveira et al. 2005). Further studies demonstrated that the small soluble PrPTSE oligomers are also the agents of neurotoxicity (Kazlauskaite et al. 2005; Minaki et al. 2009; Sandberg et al. 2011; Simoneau et al. 2007). Early neurotoxic molecular disturbances have been implicated in the impairment of the ubiquitin/proteasome system (Deriziotis et al. 2011; Kristiansen et al. 2007; McKinnon et al. 2016; Thibaudeau et al. 2018) and persistent translational repression in global proteins by activating the unfolded protein response (UPR) through increased phosphorylated translation initiation factor 2α (eIF2α-P) (Halliday et al. 2015; Moreno et al. 2012). Moreover, several studies show impairment of autophagy as a mechanism of neurotoxicity in PrD, with the enhancement of autophagy promoting clearance of intracellular PrPTSE deposits (Cortes et al. 2012; Thellung et al. 2018; Xu et al. 2012). Abnormal autophagic activation and neuronal apoptosis were also observed following treatment with highly toxic misfolded prion protein, with the underlying mechanisms including severe nicotinamide adenine dinucleotide (NAD+) starvation followed by ATP depletion (Zhou et al. 2015). In addition, p38 mitogen-activated protein kinase (MAPK) synaptic degeneration–associated neurotoxicity mechanisms were detected upon treating hippocampal neurons with PrPTSE. An inhibition of this pathway reversed the neurodegeneration process (Fang et al. 2018). A very recent study with an in vivo spontaneous model of TSE showed a pathogenic mechanism that accounted for endoplasmic reticulum and proteasomal stress with elevated levels of protein kinase RNA-like endoplasmic reticulum kinase (PERK), immunoglobulin heavy chain-binding protein (BiP), protein disulphide isomerase (PDI) and ubiquitin at both preclinical and clinical mice (Otero et al. 2021). Together, this indicates that the neurodegenerative processes of PrPTSE extend beyond misfolded protein aggregation and include dysregulated cellular metabolism, signalling pathways and proteostasis mechanisms, highlighting the multifaceted nature of PrD.
PrPTSE conversion and trafficking
The molecular mechanisms governing the autocatalytic conversion of PrPC into PrPTSE are still unclear. Hydrogen–deuterium exchange-coupled mass spectrometry demonstrated the region spanning residues from 80 to 90 to the C-terminal end undergoing refolding during the conversion of PrPC into PrPTSE (Smirnovas et al. 2011). Early insights into this process suggested the conversion of PrPC into an intermediate product (PrP*) (Cohen et al. 1994) or second product by redox reactions to cross the energy barrier for PrPTSE formation (Lee and Eisenberg 2003). PMCA demonstrated that co-factor molecules like RNA or phosphatidylethanolamine (PE) play crucial roles in regulating the conformation, infectivity and strain properties of PrPTSE (Deleault et al. 2012; Supattapone 2014). A recent study using PMCA supported the critical role of cofactors in the conversion process, which required glycosaminoglycans (GAGs), including heparan sulphate and the analogue, heparin (Imamura et al. 2016). Altogether, these studies suggest that the refolding of PrPC in the presence of seed PrPTSE happens at the C-terminal region of PrPC and requires environmental host factors or cofactors assisting the process.
Mechanisms of PrPTSE uptake are poorly understood due to the difficulty in differentiating PrPTSE from PrPC. A growing body of in vitro studies examining major interacting partners demonstrated that laminin receptor protein (LRP) (Gauczynski et al. 2006) and low-density lipoprotein receptor-related protein (LRP1) (Jen et al. 2010) may take part in PrPTSE uptake, whereas PrPC (Greil et al. 2008) and GAGs (Wolf et al. 2015) may not be crucial for this process. A recent study demonstrated that internalisation of PrPTSE is followed by trafficking through the endo-lysosomal pathway and endocytic recycling complex (Yamasaki et al. 2014). Lysosomes have been designated as the significant disintegration site of the internalised PrPTSE (Choi et al. 2014; Krejciova et al. 2014). The detection of nascent formed PrPTSE residing in the amyloidogenic fibrillar strings on the cell surface suggested a potential role of the plasma membrane during PrPTSE trafficking and conversion (Rouvinski et al. 2014). Another study using immunofluorescence and electron microscopy supported the plasma membrane and endolysosomal compartments as potential conversion sites, with extracellular trafficking occurring via exosomes, a specific subclass of EVs (Veith et al. 2009). A subsequent study demonstrated the reduction and increase of PrPTSE content when blocking MVB maturation and enhancing cargo recycling through the MVB, respectively (Yim et al. 2015). Experiments involving inhibition of the neutral sphingomyelinase pathway and stimulating exosome production demonstrated decreases in intercellular PrPTSE infectivity and increased PrPTSE content, respectively (Guo et al. 2016). Together, these studies indicate that the MVB could be a promising PrPTSE conversion site, and exosomes could play a significant role in intercellular infectivity promoting the propagation of the disease.
PrPTSE spread throughout the body
The various aetiologies of PrD range from eating BSE-infected meat products in vCJD to corneal grafting or blood transfusion in iCJD, indicating that PrPTSE spreads from the periphery to the brain. Following intraperitoneal injection of mice with scrapie prion, infection was initially found in the lymphoreticular system. The spleen was the major accumulation site before reaching the brain (Dickinson and Fraser 1969a, 1969b). Moreover, in many cases, PrPTSE has been found to accumulate in the follicular dendritic cells of lymphoid tissues (Jeffrey et al. 2000; Kitamoto et al. 1991). Subsequent experiments involving functional loss or inactivation of follicular dendritic cells resulted in the delay of neuroinvasion as well as decreased susceptibility to scrapie (Mabbott et al. 2000, 2003). Recently, intracerebral inoculation of interspecies PrPTSE in ovine and human transgenic mice demonstrated preferential PrPTSE replication in splenic versus neuronal tissues (Beringue et al. 2012).
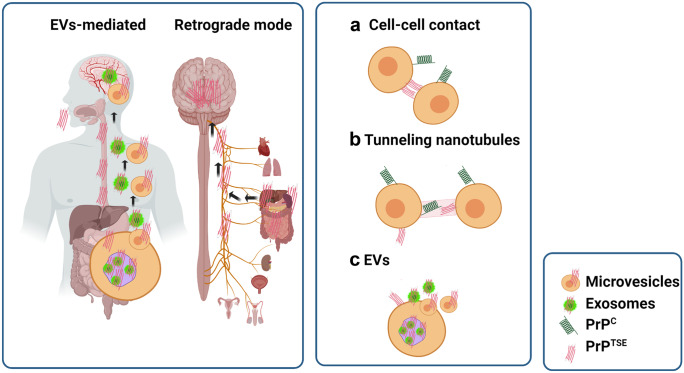
However, the mechanisms facilitating PrPTSE spread from different regions of the body to the brain remain largely unknown. One potential route supported by immunohistochemical evidence suggests that PrPTSE undergoes retrograde transmission in vagus and splanchnic nerves (McBride et al. 2001). In addition, the molecular mechanism underlying the intercellular prion spread has been proposed to primarily involve (1) direct cell-to-cell contact (Kanu et al. 2002; Paquet et al. 2007), (2) tunnelling nanotubes (Gousset et al. 2009; Langevin et al. 2010), (3) GPI painting (Baron et al. 2002) and (4) extracellular vesicles (EVs) (Fevrier et al. 2004; Guo et al. 2016; Mattei et al. 2009; Yim et al. 2015) (Fig. 3).
Fig. 3.
Schematic diagram showing different mechanisms of prion spread. Possible mechanisms of pathogenic prion protein (PrPTSE) spread to the brain from distal organs include extracellular vesicle (EV)–mediated transfer (1) or retrograde transfer across vagus and splanchnic nerves (2). The intercellular PrPTSE transfer involves cell–cell contact a, tunnelling nanotubules b and EVs c. This figure was generated using Biorender
EVs and their association with PrPC and PrPTSE
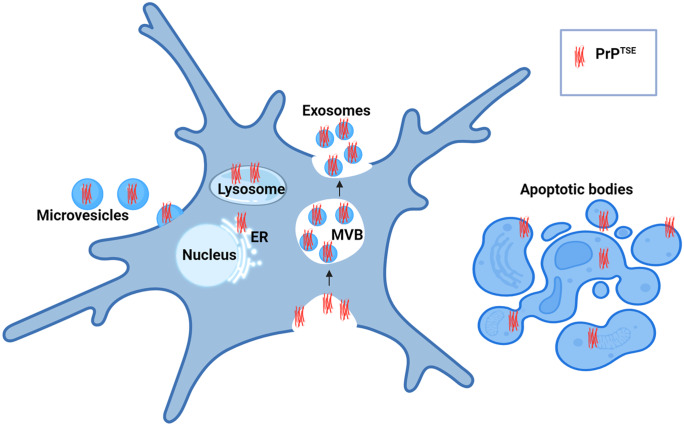
EVs are small and non-synaptic, with significant biological and pathological functions. They carry biological macromolecules, including proteins and genomic material, from cells through the extracellular fluids. Both eukaryotic and prokaryotic cells produce EVs. These are secreted by all cell types in the nervous system, becoming increasingly studied as a new method of intercellular communication and as a source of disease biomarkers. EVs include apoptotic bodies, microvesicles (also called ectosomes) and exosomes classified based on biogenetic pathway and size. Apoptotic bodies (500–5000 nm) arise from the cell fragmentation during apoptosis, microvesicles (200–1000 nm) from outward budding and shedding from the plasma membrane, and exosomes (40–200 nm) which are released intercellularly following production via the endosomal pathway and fusion of the MVB to the plasma membrane (Kalra et al. 2012; Thery et al. 2009) (Fig. 4).
Fig. 4.
Schematic diagram showing extracellular vesicle (EV) generation and pathogenic prion protein (PrPTSE) localisation in EVs. Small EVs (exosomes; < 200 nm) are generated via endosomal pathways when the multivesicular bodies (MVBs) fuse with the plasma membrane. These carry several classes of marker protein including tetraspanins, endosomal sorting complex required for transport (ESCRT) proteins, neutral sphingomyelinase 2 (nSMase2), heat shock proteins (HSPs) and integrins as defined in the Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines (Théry et al. 2018). Microvesicles are generated via blebbing or shedding from the plasma membrane, and apoptotic bodies are generated via cellular fragmentation in cells undergoing apoptotic cell death. Studies have demonstrated PrPTSE localisation in the plasma membrane, endolysosomal system including the endoplasmic reticulum (ER), and EVs
As described above (see Structure of PrPTSE), the evidence for packaging of prion protein in the EVs provides a novel mechanism of prion-induced intercellular communication. In a recent study, EVs derived from the brain demonstrated the presence of enriched prion protein which was further increased after transient ischaemia (Brenna et al. 2020). Moreover, in the same study where brain EVs were isolated from wild-type and PrPC knockout mice, EV uptake from the PrPC knockout model by primary neurons, astrocytes and microglia was significantly faster and more efficient. This indicates that the association of PrPC on the EVs plays a vital role in the physiological and pathophysiological conditions that could be targeted for therapeutic roles. A new study targeting astroglial communication and the movement of EVs along the neuronal surface revealed that a hydrophilic interaction of EV prion protein and neuronal prion protein is required for this movement in the majority of EVs (D'Arrigo et al. 2021). This association of EVs and prion protein for the neuron surface movement reveals the importance in intercellular communication where EVs can move across the axonal surface to reach the target sites. In addition, PrPC-associated EVs isolated from human plasma promote anti-inflammatory effects on LPS-induced macrophages (Mantuano et al. 2022). These studies collectively reveal a multifunctional role of PrPC-associated EVs in processes including EV uptake mechanisms, movements and the intercellular communications. Thus, the conversion of PrPC into PrPTSE would suggest a significant impact on EVs’ biophysical activities for intercellular communications.
Initial studies demonstrating exosomes could carry PrPTSE capable of infectivity transfer was shown in cell model isolated exosomes with immunogold labelling on guanidium-treated samples to specifically probe PrPTSE (Fevrier et al. 2004; Leblanc et al. 2006). A subsequent study isolated exosomes from infected neuronal cells to demonstrate in vitro and in vivo PrPTSE association with exosomes and the ability of these to transfer infectivity (Vella et al. 2007). The isolated exosomes containing PrPTSE were shown to transfer infectivity to healthy neuronal cells and non-neuronal cells in culture, indicating that infectivity transfer is possible across different cell types. Moreover, the inoculation of mice with these exosomes containing PrPTSE resulted in the development of clinical prion disease. While EVs are present in all kinds of biofluids, detection of prion infectivity in the blood (Concha-Marambio et al. 2016), milk (Gough et al. 2009; Maddison et al. 2009), urine (Gonzalez-Romero et al. 2008), cerebrospinal fluid (Foutz et al. 2017; Murayama et al. 2014), and EVs isolated from plasma (Cervenakova et al. 2016; Saá et al. 2014) suggests that EVs could be a significant route of disease spread, travelling great distances within the body. Very recently in cases of sCJD, PrPTSE was found to accumulate through regions of the eye, suggesting that tear EVs in prion-infected patients could also potentially carry prion infectivity (Orrù et al. 2018). Using in vitro models, cellular prion infectivity and exosomal release of prion infectivity were found to be associated with the endosomal sorting complexes required for transport (ESCRT)-dependent and -independent pathways of exosome release (Vilette et al. 2015). In addition, the transgenic RK13 cell line expressing ovine, mouse and vole PrPC was used to show different prion strains that exhibit specific differential release of PrPTSE associated with exosomes (Arellano-Anaya et al. 2015). This study demonstrated that most prion infectivity was associated with the exosomes from the prion-infected conditioned media, and ovine 127S infected RK13 secreted 20- to 40-fold more PrPTSE in exosomes than exosomes murine 22L and vole prions. Together, these studies indicate that EVs may be a significant source of prion infectivity and highlight how understanding the mechanisms of prion EVs’ cargo loading and cellular uptake could provide novel insight into the dysregulated biochemical functions and downstream disease processes.
EVs as a source of biomarkers for prion disease
EVs are regarded as a source of biomarkers because of their superior properties as a storage unit for various biochemical markers like nucleic acids, proteins and lipids. Moreover, their accessibility from biofluids makes them an attractive candidate for minimally invasive diagnostic purposes. EVs extracted from the blood plasma and serum are highly enriched with microRNA (miRNA), and many have predicted targets in crucial neuronal signalling pathways. This suggests that EVs could carry brain-associated miRNAs and into the blood (Cheng et al. 2014), an idea supported by studies isolating neural-derived EVs in the blood using neural cell adhesion molecule L1 (L1CAM) which is highly expressed in the brain (Fiandaca et al. 2015; Kapogiannis et al. 2015). Further evidence of brain EVs detected in the blood includes EVs isolated from blood samples of mice that have received human glioma transplants (García-Romero et al. 2017). In addition, brain tissue–derived EV isolation can enhance the precision of diagnosis related to the affected region when correlated together with the blood-based differential miRNAs and proteins in EVs for biomarker discovery (Cheng et al. 2021).
Selective packaging of miRNAs in EVs
Small non-coding miRNA (20–22 nucleotides long) play key roles in gene regulation either by inducing mRNA degradation or repression of mRNA translation. A plethora of miRNAs are present in the brain, indicating that complex physiological and neuronal development functions of miRNAs are more enriched in the brain regions, which shows the complex physiological functions of the brain (Sempere et al. 2004; Bak et al. 2008). miRNAs function with a dual nature consisting of divergent actions, meaning single miRNAs can target regulation of multiple genes, and/or convergent actions, where two or more miRNAs target the same untranslated region (UTR) (Lukiw and Alexandrov 2012). Many experiments have shown the involvement of miRNA in cellular stress-induced neurodegeneration together with the presence of toxic substances like reactive oxygen species (ROS) and polyglutamine (Bilen et al. 2006; Lukiw and Pogue 2007). Moreover, miRNA stability is essential for neuronal survival, with ablation of the miRNA generating enzyme Dicer in mouse Purkinje cells causing progressive loss of miRNA, resulting in slow cerebellar degeneration (Schaefer et al. 2007).
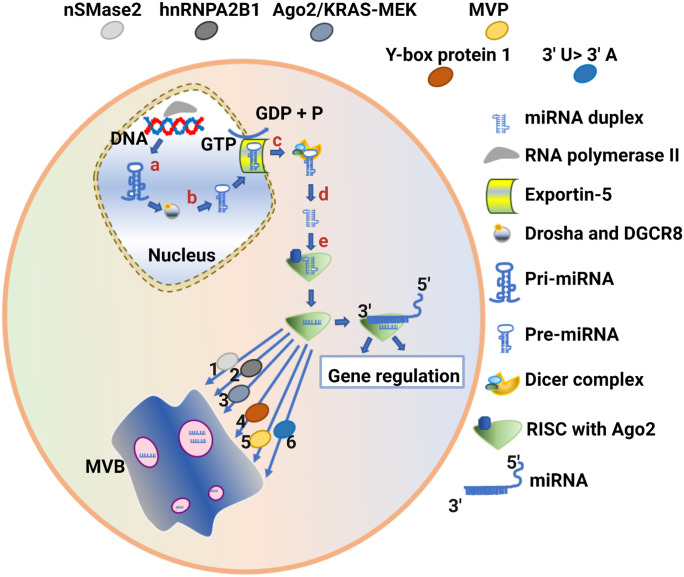
Potential mechanisms underlying miRNA packaging into EVs have been the subject of intense scrutiny in several recent studies. Knockdown of neutral sphingomyelinase 2 (nSMase2), a regulator of ceramide biosynthesis, reduced exosomes as well as miRNA secretion, an effect that was reversed following overexpression (Kosaka et al. 2010). Furthermore, nSMase2-dependent regulation of exosomal miRNAs from cancer cells was capable of promoting angiogenesis and metastasis (Kosaka et al. 2013). However, the mechanism of nSMase2 regulation of miRNA packaging into exosomes has remained elusive. Specific sequence motifs in miRNA are now known to interact with RNA binding proteins, a mechanism that both prevents degradation and acts as a vector for cellular sorting machinery. In its sumoylated form, the heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) recognises and binds the 3′ portion of miRNA containing the GGAG motif, which controls the loading of miRNA into exosomes (Villarroya-Beltri et al. 2013). Similarly, knockout or knockdown of Argonaute2 (Ago2) in cell models reduced preferentially exported exosomal miRNAs such as miR-150, miR-100 and let-7a (Guduric-Fuchs et al. 2012). The sorting of Ago2 into exosomes was further shown to be regulated by Kirstan rat sarcoma-mitogen-activated protein kinase kinase (KRAS-MEK) signalling, with inhibition of this signalling increasing sorting of Ago2 into exosomes (McKenzie et al. 2016). The Y-box protein 1 is another candidate RNA binding protein that potentially contributes to the selective packaging of miRNA into exosomes (Shurtleff et al. 2016). Shurtleff and colleagues (2016) demonstrated that Y-box protein 1 is co-packaged with synthetic miR-223-biotin in a cell-free reaction system captured using streptavidin-coated beads and detected using streptavidin tandem-mass spectrometry. Similarly, major vault protein (MVP) was captured from exosomal lysate with a bead-based streptavidin–biotin-miR-193a system and detected with matrix-assisted laser desorption ionisation-time of flight (MALDI-TOF) mass spectrometry, suggesting that MVP binds exosomal miR-193a and may be another candidate for RNA packaging (Teng et al. 2017). These studies highlight the importance of the subcellular distribution, localisation and transport of RNA-binding proteins in the process of miRNA binding and loading into EVs. While these studies have generally focused on RNA binding proteins, there is also evidence that RNA can undergo post-transcriptional modifications, ultimately altering its functional distribution and degradation patterns (Roundtree et al. 2017). Not surprisingly, RNA sequencing identified 3′ uridylated forms of miRNA that were found enriched in exosomes while 3′ adenylated miRNA forms were enriched in cells (Koppers-Lalic et al. 2014). Together, this highlights the diversity and complexity of mechanisms required to coordinate miRNA packaging and distribution into EVs (Fig. 5).
Fig. 5.
Schematic diagram showing microRNA (miRNA) biogenesis and sorting into exosomes. The alphabetical numbering shows the miRNA biogenesis pathway; a primary miRNA (Pri-miRNA) is synthesised in the nucleus by RNA polymerase II; b RNase III enzyme (Drosha) and DiGeorge syndrome critical region 8 (DGCR8) cofactor cleave Pri-miRNA into precursor miRNA (pre-miRNA); c pre-miRNA is exported to the cytoplasm by Exportin-5; d mature miRNA duplex is produced after cleavage of pre-miRNA by RNase III enzyme (Dicer); e RNA-induced silencing complex (RISC) produces single-stranded miRNA and transports it to the upstream mRNA sequence for its functional repression. The selective sorting of miRNA into exosomes has been demonstrated through many RNA binding proteins, including (1) neutral sphingomyelinase 2 (nSMase2), (2) heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), (3) Argonaute2 (Ago2), (4) Y-box protein 1 and (5) major vault protein (MVP). (6) The specific enrichment of 3′ uridylated (3′ U) miRNAs has been found in exosomes and 3′ adenylated (3′ A) miRNAs have been found in cells. This figure was generated using Biorender
Potential miRNA biomarkers in prion disease
Initial attempts to determine the role of miRNA in neurodegeneration related to prion infection were performed in mouse brains infected with mouse-adapted scrapie. These experiments identified miRNAs including miR-342-3p, miR-320, let-7b, miR-328, miR-128, miR-139-5p and miR-146a that were significantly upregulated while miR-338-3p and miR-337-3p were significantly downregulated. Moreover, bioinformatics and biochemical analysis showed that these miRNAs have functions in neuronal degradation and disease progression (Saba et al. 2008). A similar study to characterise deregulation of miRNA that potentiates the pathogenesis in human PrD was performed in BSE-infected Cynomolgus macaques. Microarray analysis demonstrated that hsa-miR-342-3p and hsa-miR-494 were significantly upregulated in brain samples (Montag et al. 2009). More recently, a study using mice infected with 139A − , ME7 − and S15 scrapie strains found two novel miRNAs, miR-2 and miR-20, downregulated by all three prion strains in the infected brain samples (Gao et al. 2016). In the same study, 14 commonly decreased and 22 commonly increased miRNAs were detected in the brain samples of all three prion strain types. Such common deregulated miRNAs indicate similar disease pathogenesis mechanisms and highlight how miRNA could act as a potential biomarker candidate.
One of the first studies that showed the diagnostic potential of EVs miRNA was carried out in prion-infected mouse hypothalamic neuronal cell cultures (Bellingham et al. 2012). This study observed upregulation of let-7i, miR-21 and downregulation of miR-146a in both prion-infected neuronal cells and exosomes secreted by these cells, whereas let-7b, miR-128a, miR-222, miR-29b, miR-342-3p and miR-424 were significantly upregulated only in exosomes (Bellingham et al. 2012). A recent prion EV miRNA biomarker study consisted of a longitudinal analysis of miRNA on M1000 prion–infected mice thalamus and serum EVs of pre-clinical and clinical samples (Cheng et al. 2021). Three potential miRNA biomarkers, miR-1a-3p, miR-181a-5p and miR-142-3p, were deregulated in both thalamus and serum EVs. Furthermore, 17 miRNAs that were differentially expressed in mice serum EVs were validated in a second independent cohort of sCJD patient serum samples. Bioinformatics predicted that the best combination of four miRNAs as a biomarker consisted of miR-423-3p, miR-101-3p, miR-1306-5p and miR-142-3p, which predicted sCJD with an area under the curve (AUC) of 0.800 (85% sensitivity and 66.7% specificity). Another recent study of naturally infected sheep with scrapie demonstrated significant upregulation of miR-21-5p in both plasma EVs and total CSF whereas other promising miRNAs significantly upregulated in the total CSF included miR-342-3p, miR-146a-5p and miR-128-3p (López-Pérez et al. 2021). Further work is required to test the consistency and role of these EV-associated miRNAs in PrD. However, the appearance of similar miRNA across different types/strains/species of PrD is highly encouraging and the utilisation of these in combination for use as a biomarker has enormous potential as a diagnostic tool in PrD.
Deregulated mitochondrial biomarkers in prion disease
After more than three decades of research following the discovery of the proteinaceous nature of PrD, several deregulated proteins have been identified. Despite discovering several proteins involving various strains and models of prion disease, no early disease-associated protein biomarker has yet been identified. Proteins that have shown potential as prion biomarkers include 14–3-3 and tau from CSF (Hsich et al. 1996; Otto et al. 2002). More recent studies that utilise CSF biomarkers have established that increases in neurofilament light chain and α-synuclein can differentially diagnose sCJD among various neurodegenerative diseases (Llorens et al. 2017; Zerr et al. 2018).
Across different models of PrD, the vast majority of deregulated proteins have been found with the application of mass spectrometry. Global proteomic analysis discovered that PrD-associated deregulated proteins fall into various functional subgroups, including protein folding, cell death, synaptic dysfunction, ion transport, oxidative stress, immune regulation and energy metabolism. One such study on mouse-adapted scrapie prion strain (22L)–infected N2a cells overexpressing murine prion protein demonstrated upregulation in the redox chaperone, protein disulphide isomerase (PDI) (Provansal et al. 2010). The majority (41.5%) of differentially expressed proteins identified in this study were involved in energy metabolism, including upregulation of the oxidative phosphorylation protein, ATP synthase. Another proteomics study on sCJD patient CSF samples demonstrated that the highest percentage of deregulated proteins, accounting for 22.10% of the differential expression, was involved in cellular metabolism (Wang et al. 2017). In addition, tricarboxylic acid (TCA) cycle proteins were also discovered, including succinate dehydrogenase subunit A (SDHA), aconitase 2 (ACO2) and malate dehydrogenase 2 (MDH2). Proteomic profiling of brain homogenates from C57BL/10SnJ mice infected with mouse-adapted ovine scrapie strain (RML) revealed increased dysfunction of the mitochondrial proteome, which was associated with neurodegeneration (Moore et al. 2014). This mitochondrial-associated apoptosis was related to elevated levels of Mitofilin, heat shock protein family A (Hsp70) member 9 (HSPA9), apoptosis-inducing factor 1 (AIF1) and various other calcium-regulated mitochondrial changes. One of the top pathways discovered in the first human PrD proteome analysis study identified fatty acid elongation in mitochondria, including deregulated enoyl-CoA hydratase, short chain 1 (ECHS1) (Shi et al. 2015). This brain region–specific proteomic analysis in different human PrD demonstrated higher cerebellum pathogenesis compared to the cortex region. Moreover, a recent proteomics study on sCJD patients’ cerebellum discovered reactive oxygen species (ROS) scavenger protein deglycase DJ-1 upregulation only in VV2 subtype, with upregulation at the mRNA level in both MM1 and VV2 subtypes (Tahir et al. 2018). Other mitochondrial associated proteins that were identified with this proteomic analysis included apoptosis-inducing factor mitochondria associated 1 (AIFM1), dihydrolipoamide S-succinyltransferase (DLST), translocase of outer mitochondrial membrane 70A (TOMM70A) and succinate-CoA ligase ADP-forming subunit beta (SUCLA2). Together, this suggests that mitochondrial dysfunction may be a primary driver of PrD pathophysiology, potentially underlying key metabolic differences contributing to neurodegeneration. The association of several key metabolic enzymes responsible for the generation of ATP suggests PrD likely disrupts ATP production and cellular energy homeostasis. Moreover, mitochondrial stress, particularly through the oxidative phosphorylation chain, likely results in redox dyshomeostasis and cellular oxidative stress, a key player in the neurodegenerative processes associated with PrD.
EV mitochondrial biomarkers
Neurodegeneration related to mitochondrial dysfunction has been extensively studied in several neurodegenerative diseases, including PrD. However, investigating mitochondrial mechanisms of neuronal cell death or its biochemical and biological profiles is still lacking in PrD models compared to neurodegenerative diseases like Parkinson’s disease (PD) and Alzheimer’s disease (AD). An early study of scrapie-infected hamster brain cerebral cortex transmission electron microscopy images depicted alterations in mitochondrial morphology and depletion of mitochondrial matrix and cristae (Choi et al. 1998). In addition, the same study demonstrated a deficiency of various mitochondrial enzymes, including manganese superoxide dismutase (Mn-SOD), cytochrome c oxidase (complex IV) and ATPase (complex V). A more recent study validated mitochondrial respiratory chain deficiency in human sCJD brain temporal cortex with immunohistochemistry, demonstrating a loss of all mitochondrial complexes (I–V) (Flønes et al. 2020). Several further studies have investigated neuronal death linked to mitochondrial dysfunction in an in vitro model system of PrD. Early co-culture experiments using primary cortical neurons together with scrapie-infected N2a cells demonstrated mitochondrial clustering in the perinuclear region of the primary cells (Resenberger et al. 2011b). A recent study isolated mitochondrial fractions from the scrapie SMB-S15 cell model and scrapie 139A- and ME7-infected mouse brains and demonstrated increased expression of phosphatase and tensin homolog (PTEN)-induced kinase1 (PINK1) and parkin in the infected cells and tissues (Gao et al. 2020). In addition, immunohistochemical analysis of various brain sections in this study observed the activation of mitophagy by PrPTSE through the colocalisation of PINK1 and parkin with PrPTSE. As noted above, this further illustrates the importance of mitochondrial dysfunction in the pathogenesis of PrD, with the activation of mitophagy highlighting that mitochondria play an active role in the pathogenesis of PrD. Further study is required to investigate the mechanisms underlying the cellular localisation of PrPTSE to the mitochondrial membrane prior to mitochondrial dysfunction and degradation.
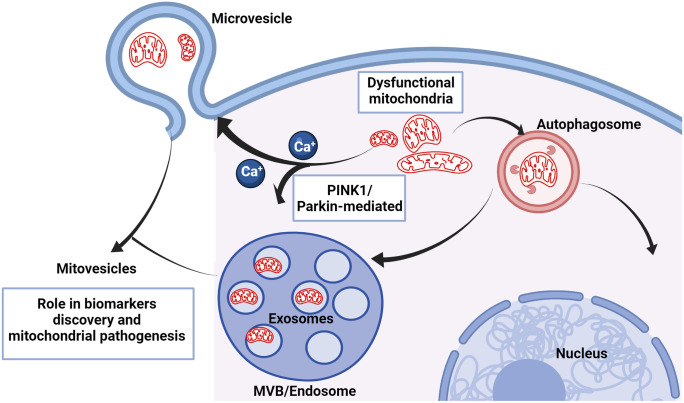
Interestingly, a novel discovery suggests that EVs may act as potential mitochondrial carriers, including whole mitochondria or mitochondrial fragments. The first clear ultrastructure of highly purified small EVs utilised cryo-electron tomography and discovered EVs with multiple membrane structures (Coleman et al. 2012). Furthermore, the EVs isolated from prion-infected and non-infected GT1-7 cells had different ratios of single membrane to double and triple membrane subpopulations. The discovery of double and triple membrane EVs could suggest that EVs incorporate other membranous organelles during their biogenesis, including mitochondria. In addition, PINK1/parkin are known regulators of intraluminal vesicle (ILV) formation, exosomes release, and the trafficking and association of mitochondria to the endosomal system (McLelland et al. 2016; Song et al. 2016). As observed in PrD, mitochondrial depolarisation causes calcium ion influx in the cytosol, likely impacting EV biogenesis by promoting calcium ion-induced membrane blebbing or MVB fusion to the plasma membrane (Record et al. 2018) (Fig. 6). Cells undergoing mitophagy potentially shuttle the entire mitochondria to microvesicle-sized EVs, a phenomenon observed by several different studies with electron microscopy images (Leermakers et al. 2020; Phinney et al. 2015). Strikingly, one recent study imaged mitochondrial fragments inside small EVs isolated from astrocytes treated with Aβ aggregates (Kim et al. 2020). In the same study, several mitochondrial coding and non-coding RNA biomarkers, including ND1-6, were found elevated in EVs isolated from plasma of AD patients. Furthermore, brain-derived EVs isolated from Down’s syndrome patients contain a different subpopulation of EVs, termed mitovesicles, containing a vast array of mitochondrial constituents (D’Acunzo et al. 2021). Taken together, these observations of mitochondrial dysfunction in PrD and the implications of mitochondrial systems in EV biogenesis highlight a new avenue in the discovery of novel mitochondrial associated EV biomarkers and treatment strategies.
Fig. 6.
Schematic diagram showing generation of extracellular vesicles (EVs) containing mitochondria (mitovesicles). Dysfunctional or depolarised mitochondria in prion disease evoke an imbalance in calcium ion concentration in the cytosol, consequently inducing the biogenesis of multivesicular bodies and microvesicles. In addition, prion-induced mitophagy with phosphatase and tensin homolog (PTEN)–induced kinase1 (PINK1)/Parkin plays a role in the association of mitochondria to the multivesicular body (MVB) to generate mitovesicles. This figure was generated using Biorender
EVs as prion therapeutics
EVs are both a protective carrier of biological molecules produced by every tissue and a functional body that delivers a signal to the recipient cells. This functional aspect of EVs has become an intense area of research due to the promise of EVs as a potential disease therapeutic strategy. For example, the use of mesenchymal stem cell (MSC)–derived EVs from human placenta intravenously delivered to multiple sclerosis mice demonstrated increased motor abilities, reduced DNA damage and increased myelination (Clark et al. 2019). Another study utilised intra-arterial injection of MSC-derived EVs from human bone marrow to induce an immunomodulatory effect and reduce neuroinflammation in rats with induced focal brain injury (Dabrowska et al. 2019). The ability of EVs injected into the blood to evoke functional effects in the brain across the blood–brain barrier (BBB) makes these an attractive therapeutic candidate for delivering pharmacological treatments capable of targeting PrPC directly to ameliorate pathogenesis of PrD.
In support of this, human neural stem cell (hNSC)–derived EVs injected into the bloodstream ameliorated neuroinflammation and increased cognitive function in immunocompetent mice (Leavitt et al. 2020). Further investigation of the protective effects of NSCs-EVs led to the discovery of the key player, miR-124, in NSCs-EVs cargo responsible for attenuating brain microglial activation. This study suggests that EVs can be engineered for treatment by transfecting cells with miRNA of interest to enrich EV cargo containing miRNA of therapeutic interest. A recent study with this proof of concept demonstrated that astrocyte-derived EVs isolated from cells transfected with miR-29 mimic ameliorated brain ischaemia–reperfusion injury via nuclear factor kappa B/nucleotide-binding domain and leucine-rich repeat-containing family, pyrin domain containing 3 (NF-κB/NLRP3) downregulation in rat brain (Liu et al. 2021). Using suitable candidate miRNA targeting PrPC, this technique would be highly relevant in the treatment of PrD as it is both easily scalable and less invasive. A promising candidate recently shown to increase the life expectancy of PrD animal models is the newly developed prion protein reducing antisense oligonucleotides (ASOs) (Minikel et al. 2020). The ability of ASOs downregulating prion protein in this study reversed the disease phenotype and increased survival across various prion strains in a dose-dependent manner. Few challenges of the nucleic acid–based therapeutic intervention are their inefficient biodistribution and susceptibility to breakdown. Therefore, utilising the innate nature of EVs with higher bioavailability and targeted engineering for the functional delivery makes them a highly promising candidate. Furthermore, ASOs are very small molecules of about 10 nucleotides in length which can be engineered in the EVs by electroporation, making the delivery to the brain efficient and less invasive.
Concluding remarks
EV research is a burgeoning field elucidating the dynamic nature of intercellular communication in the local and distal areas of the body, and has opened a new horizon to decipher the fundamental biological processes in health and disease. Studies exploiting EVs as the biomarker source and their subsequent role in pathogenic and neurotoxic processes aid a better understanding of the complexity of PrD. The gaps in the study of proteomics and lipidomic cargo from the infectious EVs of several prion strains would help to reveal common neurotoxicity-related pathways. Moreover, the functional study of infectious prion EVs in communication with the glial cells can reveal these EVs’ immunomodulatory and inflammatory effects. While secondary lymphoid organs, including the spleen, are indicated as an early accumulation site for prion replication following peripheral prion infection, isolating EVs from these organs for infectivity and cargo assessment would result in better understanding of EVs’ associated roles. Future studies utilising emerging technologies to examine the nanoscale world of EVs will shed more light on this growing field, promoting strain-specific biomarker discovery for disease diagnosis and enabling the engineering of EVs for disease treatment.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Research in the Hill laboratory is supported by grants from the National Health and Medical Research Council of Australia (to A.F.H. GNT1041413; GNT1132604), and the Australian Research Council (to A.F.H. DP170102312; DP190101655).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altmeppen HC, Prox J, Krasemann S, Puig B, Kruszewski K, Dohler F, Bernreuther C, Hoxha A, Linsenmeier L, Sikorska B, Liberski PP, Bartsch U, Saftig P, Glatzel M (2015) The sheddase ADAM10 is a potent modulator of prion disease. eLife 4 [DOI] [PMC free article] [PubMed]
- Arellano-Anaya ZE, Huor A, Leblanc P, Lehmann S, Provansal M, Raposo G, Andréoletti O, Vilette D. Prion strains are differentially released through the exosomal pathway. Cell Mol Life Sci. 2015;72:1185–1196. doi: 10.1007/s00018-014-1735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babelhadj B, Di Bari MA, Pirisinu L, Chiappini B, Gaouar SBS, Riccardi G, Marcon S, Agrimi U, Nonno R, Vaccari G. Prion disease in dromedary camels, Algeria. Emerg Infect Dis. 2018;24:1029–1036. doi: 10.3201/eid2406.172007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M, Silahtaroglu A, Møller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron GS, Caughey B. Effect of glycosylphosphatidylinositol anchor-dependent and -independent prion protein association with model raft membranes on conversion to the protease-resistant isoform. J Biol Chem. 2003;278:14883–14892. doi: 10.1074/jbc.M210840200. [DOI] [PubMed] [Google Scholar]
- Baron GS, Wehrly K, Dorward DW, Chesebro B, Caughey B. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. Embo J. 2002;21:1031–1040. doi: 10.1093/emboj/21.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C. Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. J Biol Chem. 2011;286:7490–7495. doi: 10.1074/jbc.M110.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Oesch B, Scott M, Westaway D, Walchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986;46:417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Beck E, Daniel PM, Matthews WB, Stevens DL, Alpers MP, Asher DM, Gajdusek DC, Gibbs CJ., Jr Creutzfeldt-Jakob disease. The neuropathology of a transmission experiment. Brain : a Journal of Neurology. 1969;92:699–716. doi: 10.1093/brain/92.4.699. [DOI] [PubMed] [Google Scholar]
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937–10949. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, Vilotte JL, Laude H. Facilitated cross-species transmission of prions in extraneural tissue. Science (New York, NY) 2012;335:472–475. doi: 10.1126/science.1215659. [DOI] [PubMed] [Google Scholar]
- Besnoit C, Morel C. Note sur les lésions nerveuses de la tremblante du mouton. Rev Vet. 1898;23:397–400. [Google Scholar]
- Bian J, Khaychuk V, Angers RC, Fernandez-Borges N, Vidal E, Meyerett-Reid C, Kim S, Calvi CL, Bartz JC, Hoover EA, Agrimi U, Richt JA, Castilla J, Telling GC. Prion replication without host adaptation during interspecies transmissions. Proc Natl Acad Sci USA. 2017;114:1141–1146. doi: 10.1073/pnas.1611891114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, Steele AD, Toyka KV, Nave K-A, Weis J, Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13:310. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- Brenna S, Altmeppen HC, Mohammadi B, Rissiek B, Schlink F, Ludewig P, Krisp C, Schlüter H, Failla AV, Schneider C, Glatzel M, Puig B, Magnus T. Characterization of brain-derived extracellular vesicles reveals changes in cellular origin after stroke and enrichment of the prion protein with a potential role in cellular uptake. J Extracell Ves. 2020;9:1809065. doi: 10.1080/20013078.2020.1809065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Brandel JP, Preece M, Sato T. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006;67:389–393. doi: 10.1212/01.wnl.0000231528.65069.3f. [DOI] [PubMed] [Google Scholar]
- Brown P, Gibbs CJ, Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Cervenakova L, Saa P, Yakovleva O, Vasilyeva I, de Castro J, Brown P, Dodd R. Are prions transported by plasma exosomes? Transfus Apher Sci. 2016;55:70–83. doi: 10.1016/j.transci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Chen C, Dong XP. Epidemiological characteristics of human prion diseases. Infect Dis Poverty. 2016;5:47. doi: 10.1186/s40249-016-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Quek C, Li X, Bellingham SA, Ellett LJ, Shambrook M, Zafar S, Zerr I, Lawson VA, Hill AF. Distribution of microRNA profiles in pre-clinical and clinical forms of murine and human prion disease. Commun Biol. 2021;4:411. doi: 10.1038/s42003-021-01868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sharples RA, Scicluna BJ, Hill AF (2014) Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Ves 3. 10.3402/jev.v3403.23743 [DOI] [PMC free article] [PubMed]
- Chianini F, Fernández-Borges N, Vidal E, Gibbard L, Pintado B, de Castro J, Priola SA, Hamilton S, Eaton SL, Finlayson J, Pang Y, Steele P, Reid HW, Dagleish MP, Castilla J. Rabbits are not resistant to prion infection. Proc Natl Acad Sci. 2012;109:5080–5085. doi: 10.1073/pnas.1120076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SI, Ju WK, Choi EK, Kim J, Lea HZ, Carp RI, Wisniewski HM, Kim YS. Mitochondrial dysfunction induced by oxidative stress in the brains of hamsters infected with the 263 K scrapie agent. Acta Neuropathol. 1998;96:279–286. doi: 10.1007/s004010050895. [DOI] [PubMed] [Google Scholar]
- Choi YP, Head MW, Ironside JW, Priola SA. Uptake and degradation of protease-sensitive and -resistant forms of abnormal human prion protein aggregates by human astrocytes. Am J Pathol. 2014;184:3299–3307. doi: 10.1016/j.ajpath.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Zhang S, Barthe S, Kumar P, Pivetti C, Kreutzberg N, Reed C, Wang Y, Paxton Z, Farmer D, Guo F, Wang A (2019) Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells 8(12):1497 [DOI] [PMC free article] [PubMed]
- Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK. Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proc Natl Acad Sci USA. 2007;104:18946–18951. doi: 10.1073/pnas.0706522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FE, Pan KM, Huang Z, Baldwin M, Fletterick RJ, Prusiner SB. Structural clues to prion replication. Science (New York, NY) 1994;264:530. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- Coleman BM, Hanssen E, Lawson VA, Hill AF. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. Faseb J. 2012;26:4160–4173. doi: 10.1096/fj.11-202077. [DOI] [PubMed] [Google Scholar]
- Collinge J, Palmer MS, Sidle KC, Hill AF, Gowland I, Meads J, Asante E, Bradley R, Doey LJ, Lantos PL. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature. 1995;378:779–783. doi: 10.1038/378779a0. [DOI] [PubMed] [Google Scholar]
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- Concha-Marambio L, Pritzkow S, Moda F, Tagliavini F, Ironside JW, Schulz PE, Soto C (2016) Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci Transl Med 8:370ra183 [DOI] [PMC free article] [PubMed]
- Cortes CJ, Qin K, Cook J, Solanki A, Mastrianni JA. Rapamycin delays disease onset and prevents PrP plaque deposition in a mouse model of Gerstmann–Sträussler–Scheinker disease. J Neurosci. 2012;32:12396. doi: 10.1523/JNEUROSCI.6189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez LM, Nemani SK, Duque Velásquez C, Sriraman A, Wang Y, Wille H, McKenzie D, Sim VL (2021) Asymmetric-flow field-flow fractionation of prions reveals a strain-specific continuum of quaternary structures with protease resistance developing at a hydrodynamic radius of 15 nm. PLoS Pathog 17:e1009703 [DOI] [PMC free article] [PubMed]
- D’Acunzo P, Pérez-González R, Kim Y, Hargash T, Miller C, Alldred MJ, Erdjument-Bromage H, Penikalapati SC, Pawlik M, Saito M, Saito M, Ginsberg SD, Neubert TA, Goulbourne CN, Levy E (2021) Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv 7:eabe5085 [DOI] [PMC free article] [PubMed]
- D'Arrigo G, Gabrielli M, Scaroni F, Swuec P, Amin L, Pegoraro A, Adinolfi E, Di Virgilio F, Cojoc D, Legname G, Verderio C (2021) Astrocytes-derived extracellular vesicles in motion at the neuron surface: involvement of the prion protein. J Extracell Ves 10:e12114 [DOI] [PMC free article] [PubMed]
- Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. doi: 10.1186/s12974-019-1602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleault NR, Walsh DJ, Piro JR, Wang F, Wang X, Ma J, Rees JR, Supattapone S. Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. Proc Natl Acad Sci USA. 2012;109:E1938–E1946. doi: 10.1073/pnas.1206999109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriziotis P, André R, Smith DM, Goold R, Kinghorn KJ, Kristiansen M, Nathan JA, Rosenzweig R, Krutauz D, Glickman MH, Collinge J, Goldberg AL, Tabrizi SJ. Misfolded PrP impairs the UPS by interaction with the 20S proteasome and inhibition of substrate entry. Embo J. 2011;30:3065–3077. doi: 10.1038/emboj.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AG, Fraser H. Genetical control of the concentration of ME7 scrapie agent in mouse spleen. J Comp Pathol. 1969;79:363–366. doi: 10.1016/0021-9975(69)90051-6. [DOI] [PubMed] [Google Scholar]
- Dickinson AG, Fraser H. Modification of the pathogenesis of scrapie in mice by treatment of the agent. Nature. 1969;222:892–893. doi: 10.1038/222892a0. [DOI] [PubMed] [Google Scholar]
- Fang C, Wu B, Le NTT, Imberdis T, Mercer RCC, Harris DA (2018) Prions activate a p38 MAPK synaptotoxic signaling pathway. PLoS Pathog 14:e1007283 [DOI] [PMC free article] [PubMed]
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of pre-clinical Alzheimer’s disease by a profile of pathogenic proteins in neurally-derived blood exosomes: a case-control study. Alzheimer's Dementia. 2015;11:600–607.e601. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flønes IH, Ricken G, Klotz S, Lang A, Ströbel T, Dölle C, Kovacs GG, Tzoulis C. Mitochondrial respiratory chain deficiency correlates with the severity of neuropathology in sporadic Creutzfeldt-Jakob disease. Acta Neuropathol Commun. 2020;8:50. doi: 10.1186/s40478-020-00915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foutz A, Appleby BS, Hamlin C, Liu X, Yang S, Cohen Y, Chen W, Blevins J, Fausett C, Wang H, Gambetti P, Zhang S, Hughson A, Tatsuoka C, Schonberger LB, Cohen ML, Caughey B, Safar JG. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol. 2017;81:79–92. doi: 10.1002/ana.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- Gao C, Wei J, Zhang BY, Shi Q, Chen C, Wang J, Shi Q, Dong XP (2016) MiRNA expression profiles in the brains of mice infected with scrapie agents 139A, ME7 and S15. Emerg Microb Infect 5:e115 [DOI] [PMC free article] [PubMed]
- Gao L-P, Xiao K, Wu Y-Z, Chen D-D, Yang X-H, Shi Q, Dong X-P. Enhanced mitophagy activity in prion-infected cultured cells and prion-infected experimental mice via a Pink1/Parkin-dependent mitophagy pathway. ACS Chem Neurosci. 2020;11:814–829. doi: 10.1021/acschemneuro.0c00039. [DOI] [PubMed] [Google Scholar]
- García-Romero N, Carrión-Navarro J, Esteban-Rubio S, Lázaro-Ibáñez E, Peris-Celda M, Alonso MM, Guzmán-De-Villoria J, Fernández-Carballal C, de Mendivil AO, García-Duque S, Escobedo-Lucea C, Prat-Acín R, Belda-Iniesta C, Ayuso-Sacido A. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood–brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416–1428. doi: 10.18632/oncotarget.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauczynski S, Nikles D, El-Gogo S, Papy-Garcia D, Rey C, Alban S, Barritault D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis. 2006;194:702–709. doi: 10.1086/505914. [DOI] [PubMed] [Google Scholar]
- Geoghegan JC, Valdes PA, Orem NR, Deleault NR, Williamson RA, Harris BT, Supattapone S. Selective incorporation of polyanionic molecules into hamster prions. J Biol Chem. 2007;282:36341–36353. doi: 10.1074/jbc.M704447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CJ, Jr, Gajdusek DC, Asher DM, Alpers MP, Beck E, Daniel PM, Matthews WB. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science (New York, NY) 1968;161:388–389. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Petersen RB, Tabaton M, Brown P, LeBlanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, et al. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science (New York, NY) 1992;258:806–808. doi: 10.1126/science.1439789. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–3166. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough KC, Baker CA, Taema M, Maddison BC. In vitro amplification of prions from milk in the detection of subclinical infections. Prion. 2009;3:236–239. doi: 10.4161/pri.3.4.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Govaerts C, Wille H, Prusiner SB, Cohen FE. Evidence for assembly of prions with left-handed beta-helices into trimers. Proc Natl Acad Sci USA. 2004;101:8342–8347. doi: 10.1073/pnas.0402254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greil CS, Vorberg IM, Ward AE, Meade-White KD, Harris DA, Priola SA. Acute cellular uptake of abnormal prion protein is cell type and scrapie-strain independent. Virology. 2008;379:284–293. doi: 10.1016/j.virol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman BR, Dolan MA, Taubner LM, Kraus A, Wickner RB, Caughey B. Parallel in-register intermolecular beta-sheet architectures for prion-seeded prion protein (PrP) amyloids. J Biol Chem. 2014;289:24129–24142. doi: 10.1074/jbc.M114.578344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290:3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BB, Bellingham SA, Hill AF. Stimulating the release of exosomes increases the intercellular transfer of prions. J Biol Chem. 2016;291:5128–5137. doi: 10.1074/jbc.M115.684258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M, Radford H, Sekine Y, Moreno J, Verity N, le Quesne J, Ortori CA, Barrett DA, Fromont C, Fischer PM, Harding HP, Ron D, Mallucci GR (2015) Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 6:e1672 [DOI] [PMC free article] [PubMed]
- Herrmann US, Sonati T, Falsig J, Reimann RR, Dametto P, O’Connor T, Li B, Lau A, Hornemann S, Sorce S, Wagner U, Sanoudou D, Aguzzi A (2015) Prion infections and anti-PrP antibodies trigger converging neurotoxic pathways. PLoS Pathog 11:e1004662 [DOI] [PMC free article] [PubMed]
- Hill AF, Joiner S, Wadsworth JD, Sidle KC, Bell JE, Budka H, Ironside JW, Collinge J. Molecular classification of sporadic Creutzfeldt-Jakob disease. Brain : A J Neurol. 2003;126:1333–1346. doi: 10.1093/brain/awg125. [DOI] [PubMed] [Google Scholar]
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med. 1996;335:924–930. doi: 10.1056/NEJM199609263351303. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T, Tobler I. Sleep deprivation in prion protein deficient mice sleep deprivation in prion protein deficient mice and control mice: genotype dependent regional rebound. Neuro Rep. 2002;13:1–4. doi: 10.1097/00001756-200201210-00005. [DOI] [PubMed] [Google Scholar]
- Imamura M, Tabeta N, Kato N, Matsuura Y, Iwamaru Y, Yokoyama T, Murayama Y. Heparan sulfate and heparin promote faithful prion replication in vitro by binding to normal and abnormal prion proteins in protein misfolding cyclic amplification. J Biol Chem. 2016;291:26478–26486. doi: 10.1074/jbc.M116.745851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey M, McGovern G, Goodsir CM, Brown KL, Bruce ME. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol. 2000;191:323–332. doi: 10.1002/1096-9896(200007)191:3<323::AID-PATH629>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jen A, Parkyn CJ, Mootoosamy RC, Ford MJ, Warley A, Liu Q, Bu G, Baskakov IV, Moestrup S, McGuinness L, Emptage N, Morris RJ. Neuronal low-density lipoprotein receptor-related protein 1 binds and endocytoses prion fibrils via receptor cluster 4. J Cell Sci. 2010;123:246–255. doi: 10.1242/jcs.058099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borras FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Kramer-Albers EM, Lim SK, Llorente A, Lotvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-'t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sanchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vazquez J, Vidal M, Wauben MH, Yanez-Mo M, Zoeller M, Mathivanan S (2012) Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10:e1001450 [DOI] [PMC free article] [PubMed]
- Kamali-Jamil R, Vázquez-Fernández E, Tancowny B, Rathod V, Amidian S, Wang X, Tang X, Fang A, Senatore A, Hornemann S, Dudas S, Aguzzi A, Young HS, Wille H (2021) The ultrastructure of infectious L-type bovine spongiform encephalopathy prions constrains molecular models. [DOI] [PMC free article] [PubMed]
- Kanu N, Imokawa Y, Drechsel DN, Williamson RA, Birkett CR, Bostock CJ, Brockes JP. Transfer of scrapie prion infectivity by cell contact in culture. Curr Biol. 2002;12:523–530. doi: 10.1016/S0960-9822(02)00722-4. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J : Official Public Fed Am Soc Experiment Biol. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite J, Young A, Gardner CE, Macpherson JV, Venien-Bryan C, Pinheiro TJ. An unusual soluble beta-turn-rich conformation of prion is involved in fibril formation and toxic to neuronal cells. Biochem Biophys Res Commun. 2005;328:292–305. doi: 10.1016/j.bbrc.2004.12.172. [DOI] [PubMed] [Google Scholar]
- Kim KM, Meng Q, Perez de Acha O, Mustapic M, Cheng A, Eren E, Kundu G, Piao Y, Munk R, Wood WH, 3rd, De S, Noh JH, Delannoy M, Cheng L, Abdelmohsen K, Kapogiannis D, Gorospe M (2020) Mitochondrial RNA in Alzheimer’s disease circulating extracellular vesicles. Front Cell Dev Biol 8:581882 [DOI] [PMC free article] [PubMed]
- Kim Y, Lee J, Lee C. In silico comparative analysis of DNA and amino acid sequences for prion protein gene. Transbound Emerg Dis. 2008;55:105–114. doi: 10.1111/j.1865-1682.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Kimberlin RH, Walker CA. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J Gen Virol. 1978;39:487–496. doi: 10.1099/0022-1317-39-3-487. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Muramoto T, Mohri S, Doh-Ura K, Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991;65:6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Asano M, Mohri S, Kitamoto T. Cross-sequence transmission of sporadic Creutzfeldt-Jakob disease creates a new prion strain. J Biol Chem. 2007;282:30022–30028. doi: 10.1074/jbc.M704597200. [DOI] [PubMed] [Google Scholar]
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX, Würdinger T, Meijer GA, Pegtel DM. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Hoyt F, Schwartz CL, Hansen B, Artikis E, Hughson AG, Raymond GJ, Race B, Baron GS, Caughey B (2021) High-resolution structure and strain comparison of infectious mammalian prions. Molecular Cell 81(21) 4540-4551.e6 10.1016/j.molcel.2021.08.011 [DOI] [PubMed]
- Krejciova Z, De Sousa P, Manson J, Ironside JW, Head MW. Human tonsil-derived follicular dendritic-like cells are refractory to human prion infection in vitro and traffic disease-associated prion protein to lysosomes. Am J Pathol. 2014;184:64–70. doi: 10.1016/j.ajpath.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Deriziotis P, Dimcheff DE, Jackson GS, Ovaa H, Naumann H, Clarke AR, van Leeuwen FW, Menendez-Benito V, Dantuma NP, Portis JL, Collinge J, Tabrizi SJ. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell. 2007;26:175–188. doi: 10.1016/j.molcel.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Küffer A, Lakkaraju AKK, Mogha A, Petersen SC, Airich K, Doucerain C, Marpakwar R, Bakirci P, Senatore A, Monnard A, Schiavi C, Nuvolone M, Grosshans B, Hornemann S, Bassilana F, Monk KR, Aguzzi A. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464. doi: 10.1038/nature19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin C, Gousset K, Costanzo M, Richard-Le Goff O, Zurzolo C. Characterization of the role of dendritic cells in prion transfer to primary neurons. Biochem J. 2010;431:189–198. doi: 10.1042/BJ20100698. [DOI] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson VA, Vella LJ, Stewart JD, Sharples RA, Klemm H, Machalek DM, Masters CL, Cappai R, Collins SJ, Hill AF. Mouse-adapted sporadic human Creutzfeldt-Jakob disease prions propagate in cell culture. Int J Biochem Cell Biol. 2008;40:2793–2801. doi: 10.1016/j.biocel.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Leavitt RJ, Acharya MM, Baulch JE, Limoli CL. Extracellular vesicle–derived miR-124 resolves radiation-induced brain injury. Can Res. 2020;80:4266. doi: 10.1158/0008-5472.CAN-20-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc P, Alais S, Porto-Carreiro I, Lehmann S, Grassi J, Raposo G, Darlix JL. Retrovirus infection strongly enhances scrapie infectivity release in cell culture. Embo j. 2006;25:2674–2685. doi: 10.1038/sj.emboj.7601162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Eisenberg D. Seeded conversion of recombinant prion protein to a disulfide-bonded oligomer by a reduction-oxidation process. Nat Struct Biol. 2003;10:725–730. doi: 10.1038/nsb961. [DOI] [PubMed] [Google Scholar]
- Leermakers PA, Remels AHV, Zonneveld MI, Rouschop KMA, Schols A, Gosker HR. Iron deficiency-induced loss of skeletal muscle mitochondrial proteins and respiratory capacity; the role of mitophagy and secretion of mitochondria-containing vesicles. Faseb j. 2020;34:6703–6717. doi: 10.1096/fj.201901815R. [DOI] [PubMed] [Google Scholar]
- Liu X, Lv X, Liu Z, Zhang M, Leng Y (2021) MircoRNA-29a in astrocyte-derived extracellular vesicles suppresses brain ischemia reperfusion injury via TP53INP1 and the NF-κB/NLRP3 axis. Cell Mole Neurobiol [DOI] [PMC free article] [PubMed]
- Llorens F, Kruse N, Schmitz M, Gotzmann N, Golanska E, Thüne K, Zejneli O, Kanata E, Knipper T, Cramm M, Lange P, Zafar S, Sikorska B, Liberski PP, Mitrova E, Varges D, Schmidt C, Sklaviadis T, Mollenhauer B, Zerr I. Evaluation of α-synuclein as a novel cerebrospinal fluid biomarker in different forms of prion diseases. Alzheimers Dement. 2017;13:710–719. doi: 10.1016/j.jalz.2016.09.013. [DOI] [PubMed] [Google Scholar]
- López-Pérez Ó, Sanz-Rubio D, Hernaiz A, Betancor M, Otero A, Castilla J, Andréoletti O, Badiola JJ, Zaragoza P, Bolea R, Toivonen JM, Martín-Burriel I (2021) Cerebrospinal fluid and plasma small extracellular vesicles and miRNAs as biomarkers for prion diseases. Internatl J Mole Sci 22 [DOI] [PMC free article] [PubMed]
- Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott NA, Mackay F, Minns F, Bruce ME. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med. 2000;6:719. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Young J, McConnell I, Bruce ME. Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility. J Virol. 2003;77:6845–6854. doi: 10.1128/JVI.77.12.6845-6854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison BC, Baker CA, Rees HC, Terry LA, Thorne L, Bellworthy SJ, Whitelam GC, Gough KC. Prions are secreted in milk from clinically normal scrapie-exposed sheep. J Virol. 2009;83:8293. doi: 10.1128/JVI.00051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E, Azmoon P, Banki MA, Sigurdson CJ, Campana WM, Gonias SL. A soluble PrP(C) derivative and membrane-anchored PrP(C) in extracellular vesicles attenuate innate immunity by engaging the NMDA-R/LRP1 receptor complex. J Immunol. 2022;208:85–96. doi: 10.4049/jimmunol.2100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros-Angles A, Hervera A, Soriano J, Martí E, Carulla P, Llorens F, Nuvolone M, Aguzzi A, Ferrer I, Gruart A, Delgado-García JM, Del Río JA. Analysis of co-isogenic prion protein deficient mice reveals behavioral deficits, learning impairment, and enhanced hippocampal excitability. BMC Biol. 2022;20:17. doi: 10.1186/s12915-021-01203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei V, Barenco MG, Tasciotti V, Garofalo T, Longo A, Boller K, Löwer J, Misasi R, Montrasio F, Sorice M (2009) Paracrine diffusion of PrPC and propagation of prion infectivity by plasma membrane-derived microvesicles. PLOS ONE 4:e5057 [DOI] [PMC free article] [PubMed]
- McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol. 2001;75:9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15:978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon C, Goold R, Andre R, Devoy A, Ortega Z, Moonga J, Linehan JM, Brandner S, Lucas JJ, Collinge J, Tabrizi SJ. Prion-mediated neurodegeneration is associated with early impairment of the ubiquitin-proteasome system. Acta Neuropathol. 2016;131:411–425. doi: 10.1007/s00401-015-1508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland GL, Lee SA, McBride HM, Fon EA. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol. 2016;214:275–291. doi: 10.1083/jcb.201603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian M, Brethour D, Williams D, Wang H, Arnould H, Schneider B, Schmitt-Ulms G (2016) Prion protein deficiency causes diverse proteome shifts in cell models that escape detection in brain tissue. PLOS ONE 11:e0156779 [DOI] [PMC free article] [PubMed]
- Minaki H, Sasaki K, Honda H, Iwaki T. Prion protein oligomers in Creutzfeldt-Jakob disease detected by gel-filtration centrifuge columns. Neuropathology : Official Journal of the Japanese Society of Neuropathology. 2009;29:536–542. doi: 10.1111/j.1440-1789.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- Minikel EV, Zhao HT, Le J, O'Moore J, Pitstick R, Graffam S, Carlson GA, Kavanaugh MP, Kriz J, Kim JB, Ma J, Wille H, Aiken J, McKenzie D, Doh-Ura K, Beck M, O'Keefe R, Stathopoulos J, Caron T, Schreiber SL, Carroll JB, Kordasiewicz HB, Cabin DE, Vallabh SM. Prion protein lowering is a disease-modifying therapy across prion disease stages, strains and endpoints. Nucleic Acids Res. 2020;48:10615–10631. doi: 10.1093/nar/gkaa616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov A, Latawiec D, Wille H, Bouzamondo-Bernstein E, Legname G, Williamson RA, Burton D, DeArmond SJ, Prusiner SB, Peters PJ. Cytosolic prion protein in neurons. J Neurosci. 2003;23:7183. doi: 10.1523/JNEUROSCI.23-18-07183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag J, Hitt R, Opitz L, Schulz-Schaeffer WJ, Hunsmann G, Motzkus D. Upregulation of miRNA hsa-miR-342-3p in experimental and idiopathic prion disease. Mol Neurodegener. 2009;4:36. doi: 10.1186/1750-1326-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Sturdevant DE, Chesebro B, Priola SA. Proteomics analysis of amyloid and nonamyloid prion disease phenotypes reveals both common and divergent mechanisms of neuropathogenesis. J Proteome Res. 2014;13:4620–4634. doi: 10.1021/pr500329w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis AE, Bushell M, Mallucci GR. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature. 2012;485:507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y, Masujin K, Imamura M, Ono F, Shibata H, Tobiume M, Yamamura T, Shimozaki N, Terao K, Yamakawa Y, Sata T. Ultrasensitive detection of PrP(Sc) in the cerebrospinal fluid and blood of macaques infected with bovine spongiform encephalopathy prion. J Gen Virol. 2014;95:2576–2588. doi: 10.1099/vir.0.066225-0. [DOI] [PubMed] [Google Scholar]
- Nguyen XTA, Tran TH, Cojoc D, Legname G (2019) Copper binding regulates cellular prion protein function. Mole Neurobiol [DOI] [PubMed]
- Nuvolone M, Hermann M, Sorce S, Russo G, Tiberi C, Schwarz P, Minikel E, Sanoudou D, Pelczar P, Aguzzi A (2016) Strictly co-isogenic C57BL/6J-Prnp−/− mice: a rigorous resource for prion science. J Experiment Med 213:313-327 [DOI] [PMC free article] [PubMed]
- Oesch B, Westaway D, Walchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE et al (1985) A cellular gene encodes scrapie PrP 27-30 protein. Cell 40:735-746 [DOI] [PubMed]
- Orrù CD, Soldau K, Cordano C, Llibre-Guerra J, Green AJ, Sanchez H, Groveman BR, Edland SD, Safar JG, Lin JH, Caughey B, Geschwind MD, Sigurdson CJ (2018) Prion seeds distribute throughout the eyes of sporadic Creutzfeldt-Jakob disease patients. mBio 9:e02095–02018 [DOI] [PMC free article] [PubMed]
- Otero A, Betancor M, Eraña H, Fernández Borges N, Lucas JJ, Badiola JJ, Castilla J, Bolea R (2021) Prion-associated neurodegeneration causes both endoplasmic reticulum stress and proteasome impairment in a murine model of spontaneous disease. Internatl J Mole Sci 22 [DOI] [PMC free article] [PubMed]
- Otto M, Wiltfang J, Cepek L, Neumann M, Mollenhauer B, Steinacker P, Ciesielczyk B, Schulz-Schaeffer W, Kretzschmar HA, Poser S. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2002;58:192–197. doi: 10.1212/WNL.58.2.192. [DOI] [PubMed] [Google Scholar]
- Paquet S, Langevin C, Chapuis J, Jackson GS, Laude H, Vilette D. Efficient dissemination of prions through preferential transmission to nearby cells. J Gen Virol. 2007;88:706–713. doi: 10.1099/vir.0.82336-0. [DOI] [PubMed] [Google Scholar]
- Parchi P, Notari S, Weber P, Schimmel H, Budka H, Ferrer I, Haik S, Hauw JJ, Head MW, Ironside JW, Limido L, Rodriguez A, Strobel T, Tagliavini F, Kretzschmar HA. Inter-laboratory assessment of PrPSc typing in Creutzfeldt-Jakob disease: a Western blot study within the NeuroPrion Consortium. Brain Pathol. 2009;19:384–391. doi: 10.1111/j.1750-3639.2008.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly PC, Harris DA. Copper stimulates endocytosis of the prion protein. J Biol Chem. 1998;273:33107–33110. doi: 10.1074/jbc.273.50.33107. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provansal M, Roche S, Pastore M, Casanova D, Belondrade M, Alais S, Leblanc P, Windl O, Lehmann S. Proteomic consequences of expression and pathological conversion of the prion protein in inducible neuroblastoma N2a cells. Prion. 2010;4:292–301. doi: 10.4161/pri.4.4.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci. 1998;95:13363. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Race B, Phillips K, Meade-White K, Striebel J, Chesebro B. Increased infectivity of anchorless mouse scrapie prions in transgenic mice overexpressing human prion protein. J Virol. 2015;89:6022–6032. doi: 10.1128/JVI.00362-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi W, Vilette D, Guiraud P, Arlotto M, Riondel J, Laude H, Lehmann S, Favier A. Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. J Biol Chem. 2003;278:9064–9072. doi: 10.1074/jbc.M211830200. [DOI] [PubMed] [Google Scholar]
- Record M, Silvente-Poirot S, Poirot M, Wakelam MJO. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018;59:1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Muller V, Krishnan R, Vabulas RM, Kretzschmar HA, Lindquist S, Hartl FU, Multhaup G, Winklhofer KF, Tatzelt J. The cellular prion protein mediates neurotoxic signalling of beta-sheet-rich conformers independent of prion replication. Embo j. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Müller V, Krishnan R, Vabulas RM, Kretzschmar HA, Lindquist S, Hartl FU, Multhaup G, Winklhofer KF, Tatzelt J. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. Embo j. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek R, Hornemann S, Wider G, Glockshuber R, Wuthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231) FEBS Lett. 1997;413:282–288. doi: 10.1016/S0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvinski A, Karniely S, Kounin M, Moussa S, Goldberg MD, Warburg G, Lyakhovetsky R, Papy-Garcia D, Kutzsche J, Korth C, Carlson GA, Godsave SF, Peters PJ, Luhr K, Kristensson K, Taraboulos A. Live imaging of prions reveals nascent PrPSc in cell-surface, raft-associated amyloid strings and webs. J Cell Biol. 2014;204:423–441. doi: 10.1083/jcb.201308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saá P, Yakovleva O, de Castro J, Vasilyeva I, De Paoli SH, Simak J, Cervenakova L. First demonstration of transmissible spongiform encephalopathy-associated prion protein (PrPTSE) in extracellular vesicles from plasma of mice infected with mouse-adapted variant Creutzfeldt-Jakob disease by in vitro amplification. J Biol Chem. 2014;289:29247–29260. doi: 10.1074/jbc.M114.589564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Goodman CD, Huzarewich RLCH, Robertson C, Booth SA (2008) A miRNA signature of prion induced neurodegeneration. PLoS ONE 3:e3652 [DOI] [PMC free article] [PubMed]
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- Santuccione A, Sytnyk V, Leshchyns KI, Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59<sup>fyn</sup> and to enhance neurite outgrowth. J Cell Biol. 2005;169:341. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. PrPC association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell. 2004;15:4031–4042. doi: 10.1091/mbc.e03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, O'Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ulms G, Legname G, Baldwin MA, Ball HL, Bradon N, Bosque PJ, Crossin KL, Edelman GM, DeArmond SJ, Cohen FE, Prusiner SB. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. Edited by P E Wright. J Mol Biol. 2001;314:1209–1225. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Chen LN, Zhang BY, Xiao K, Zhou W, Chen C, Zhang XM, Tian C, Gao C, Wang J, Han J, Dong XP. Proteomics analyses for the global proteins in the brain tissues of different human prion diseases. Mol Cell Proteomics. 2015;14:854–869. doi: 10.1074/mcp.M114.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R (2016) Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 5:e19276 [DOI] [PMC free article] [PubMed]
- Shyng SL, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994;125:1239. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CJ, Vazquez-Fernandez E, Onisko B, Requena JR. Proteinase K and the structure of PrPSc: the good, the bad and the ugly. Virus Res. 2015;207:120–126. doi: 10.1016/j.virusres.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau S, Rezaei H, Salès N, Kaiser-Schulz G, Lefebvre-Roque M, Vidal C, Fournier J-G, Comte J, Wopfner F, Grosclaude J, Schätzl H, Lasmézas CI (2007) In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog 3:e125 [DOI] [PMC free article] [PubMed]
- Šišková Z, Reynolds RA, O’Connor V, Perry VH (2013) Brain region specific pre-synaptic and post-synaptic degeneration are early components of neuropathology in prion disease. PLOS ONE 8:e55004 [DOI] [PMC free article] [PubMed]
- Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, Surewicz WK. Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol. 2011;18:504–506. doi: 10.1038/nsmb.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Trajkovic K, Tsunemi T, Krainc D. Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J Neurosci. 2016;36:2425–2437. doi: 10.1523/JNEUROSCI.2569-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolli G, Rigoli M, Orioli S, Sevillano AM, Faccioli P, Wille H, Biasini E, Requena JR (2019) Full atomistic model of prion structure and conversion. PLoS Pathog 15:e1007864 [DOI] [PMC free article] [PubMed]
- Steele AD, Emsley JG, Ozdinler PH, Lindquist S, Macklis JD. Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA. 2006;103:3416–3421. doi: 10.1073/pnas.0511290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyach C, Jen A, Deng J, Fitzgerald KT, Frobert Y, Grassi J, McCaffrey MW, Morris R. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. Embo j. 2003;22:3591–3601. doi: 10.1093/emboj/cdg344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S. Synthesis of high titer infectious prions with cofactor molecules. J Biol Chem. 2014;289:19850–19854. doi: 10.1074/jbc.R113.511329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir W, Zafar S, Llorens F, Arora AS, Thüne K, Schmitz M, Gotzmann N, Kruse N, Mollenhauer B, Torres JM, Andréoletti O, Ferrer I, Zerr I. Molecular alterations in the cerebellum of sporadic Creutzfeldt-Jakob disease subtypes with DJ-1 as a key regulator of oxidative stress. Mol Neurobiol. 2018;55:517–537. doi: 10.1007/s12035-016-0294-4. [DOI] [PubMed] [Google Scholar]
- Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, Deng Z, Kumar A, Zhang L, Merchant ML, Yan J, Miller DM, Zhang HG. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun. 2017;8:14448. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellung S, Scoti B, Corsaro A, Villa V, Nizzari M, Gagliani MC, Porcile C, Russo C, Pagano A, Tacchetti C, Cortese K, Florio T. Pharmacological activation of autophagy favors the clearing of intracellular aggregates of misfolded prion protein peptide to prevent neuronal death. Cell Death Dis. 2018;9:166. doi: 10.1038/s41419-017-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman MLD, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DRF, Caruso S, Chamley LW, Chang Y-T, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FAW, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana Junior EF, De Wever O, del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TAP, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DCI, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano S-i, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke Ii DJ, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li ITS, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SLN, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen ENM, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BCH, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IKH, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KMA, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder Ii OL, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BWM, van der Grein SG, Van Deun J, van Herwijnen MJC, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MHM, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang J-y, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudeau TA, Anderson RT, Smith DM. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun. 2018;9:1097. doi: 10.1038/s41467-018-03509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompsett AR, Abdelraheim SR, Daniels M, Brown DR. High affinity binding between copper and full-length prion protein identified by two different techniques. J Biol Chem. 2005;280:42750–42758. doi: 10.1074/jbc.M506521200. [DOI] [PubMed] [Google Scholar]
- Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBride PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- Tycko R, Savtchenko R, Ostapchenko VG, Makarava N, Baskakov IV. The α-helical C-terminal domain of full-length recombinant PrP converts to an in-register parallel β-sheet structure in PrP fibrils: evidence from solid state nuclear magnetic resonance. Biochemistry. 2010;49:9488–9497. doi: 10.1021/bi1013134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanik DL, Surewicz KA, Surewicz WK. Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol Cell. 2004;14:139–145. doi: 10.1016/S1097-2765(04)00155-8. [DOI] [PubMed] [Google Scholar]
- Vazquez-Fernandez E, Alonso J, Pastrana MA, Ramos A, Stitz L, Vidal E, Dynin I, Petsch B, Silva CJ, Requena JR (2012) Structural organization of mammalian prions as probed by limited proteolysis. PLOS ONE 7:e50111 [DOI] [PMC free article] [PubMed]
- Vazquez-Fernandez E, Vos MR, Afanasyev P, Cebey L, Sevillano AM, Vidal E, Rosa I, Renault L, Ramos A, Peters PJ, Fernandez JJ, van Heel M, Young HS, Requena JR, Wille H (2016) The structural architecture of an infectious mammalian prion using electron cryomicroscopy. PLoS Pathog 12:e1005835 [DOI] [PMC free article] [PubMed]
- Veith NM, Plattner H, Stuermer CA, Schulz-Schaeffer WJ, Burkle A. Immunolocalisation of PrPSc in scrapie-infected N2a mouse neuroblastoma cells by light and electron microscopy. Eur J Cell Biol. 2009;88:45–63. doi: 10.1016/j.ejcb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- Vilette D, Laulagnier K, Huor A, Alais S, Simoes S, Maryse R, Provansal M, Lehmann S, Andreoletti O, Schaeffer L, Raposo G, Leblanc P. Efficient inhibition of infectious prions multiplication and release by targeting the exosomal pathway. Cell Mol Life Sci. 2015;72:4409–4427. doi: 10.1007/s00018-015-1945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth JD, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, Welch J, Stone L, Lloyd SE, Hill AF, Brandner S, Collinge J. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science (New York, NY) 2004;306:1793–1796. doi: 10.1126/science.1103932. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao D, Shah SZA, Yang W, Li C, Yang L. Proteome analysis of potential synaptic vesicle cycle biomarkers in the cerebrospinal fluid of patients with sporadic Creutzfeldt-Jakob disease. Mol Neurobiol. 2017;54:5177–5191. doi: 10.1007/s12035-016-0029-6. [DOI] [PubMed] [Google Scholar]
- Watt NT, Taylor DR, Kerrigan TL, Griffiths HH, Rushworth JV, Whitehouse IJ, Hooper NM. Prion protein facilitates uptake of zinc into neuronal cells. Nat Commun. 2012;3:1134. doi: 10.1038/ncomms2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Veterinary Record. 1987;121:419. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- Will RG, Ironside JW, Zeidler M, Estibeiro K, Cousens SN, Smith PG, Alperovitch A, Poser S, Pocchiari M, Hofman A. A new variant of Creutzfeldt-Jakob disease in the UK. The Lancet. 1996;347:921–925. doi: 10.1016/S0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- Wille H, Bian W, McDonald M, Kendall A, Colby DW, Bloch L, Ollesch J, Borovinskiy AL, Cohen FE, Prusiner SB, Stubbs G. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci U S A. 2009;106:16990–16995. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H, Michelitsch MD, Guenebaut V, Supattapone S, Serban A, Cohen FE, Agard DA, Prusiner SB. Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci U S A. 2002;99:3563–3568. doi: 10.1073/pnas.052703499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Grassmann A, Bester R, Hossinger A, Mohl C, Paulsen L, Groschup MH, Schatzl H, Vorberg I. Modulation of Glycosaminoglycans Affects PrPSc Metabolism but Does Not Block PrPSc Uptake. J Virol. 2015;89:9853–9864. doi: 10.1128/JVI.01276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tian C, Wang S-B, Xie W-L, Guo Y, Zhang J, Shi Q, Chen C, Dong X-P. Activation of the macroautophagic system in scrapie-infected experimental animals and human genetic prion diseases. Autophagy. 2012;8:1604–1620. doi: 10.4161/auto.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Baron GS, Suzuki A, Hasebe R, Horiuchi M. Characterization of intracellular dynamics of inoculated PrP-res and newly generated PrPSc during early stage prion infection in Neuro2a cells. Virology. 2014;450–451:324–335. doi: 10.1016/j.virol.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim Y-I, Park B-C, Yadavalli R, Zhao X, Eisenberg E, Greene LE. The multivesicular body is the major internal site of prion conversion. J Cell Sci. 2015;128:1434. doi: 10.1242/jcs.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar S, von Ahsen N, Oellerich M, Zerr I, Schulz-Schaeffer WJ, Armstrong VW, Asif AR. Proteomics approach to identify the interacting partners of cellular prion protein and characterization of Rab7a interaction in neuronal cells. J Proteome Res. 2011;10:3123–3135. doi: 10.1021/pr2001989. [DOI] [PubMed] [Google Scholar]
- Zanata SM, Lopes MH, Mercadante AF, Hajj GNM, Chiarini LB, Nomizo R, Freitas ARO, Cabral ALB, Lee KS, Juliano MA, de Oliveira E, Jachieri SG, Burlingame A, Huang L, Linden R, Brentani RR, Martins VR. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. Embo j. 2002;21:3307–3316. doi: 10.1093/emboj/cdf325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr I, Schmitz M, Karch A, Villar-Piqué A, Kanata E, Golanska E, Díaz-Lucena D, Karsanidou A, Hermann P, Knipper T, Goebel S, Varges D, Sklaviadis T, Sikorska B, Liberski PP, Santana I, Ferrer I, Zetterberg H, Blennow K, Calero O, Calero M, Ladogana A, Sánchez-Valle R, Baldeiras I, Llorens F. Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement. 2018;14:751–763. doi: 10.1016/j.jalz.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmézas CI. Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain : a Journal of Neurology. 2015;138:992–1008. doi: 10.1093/brain/awv002. [DOI] [PMC free article] [PubMed] [Google Scholar]