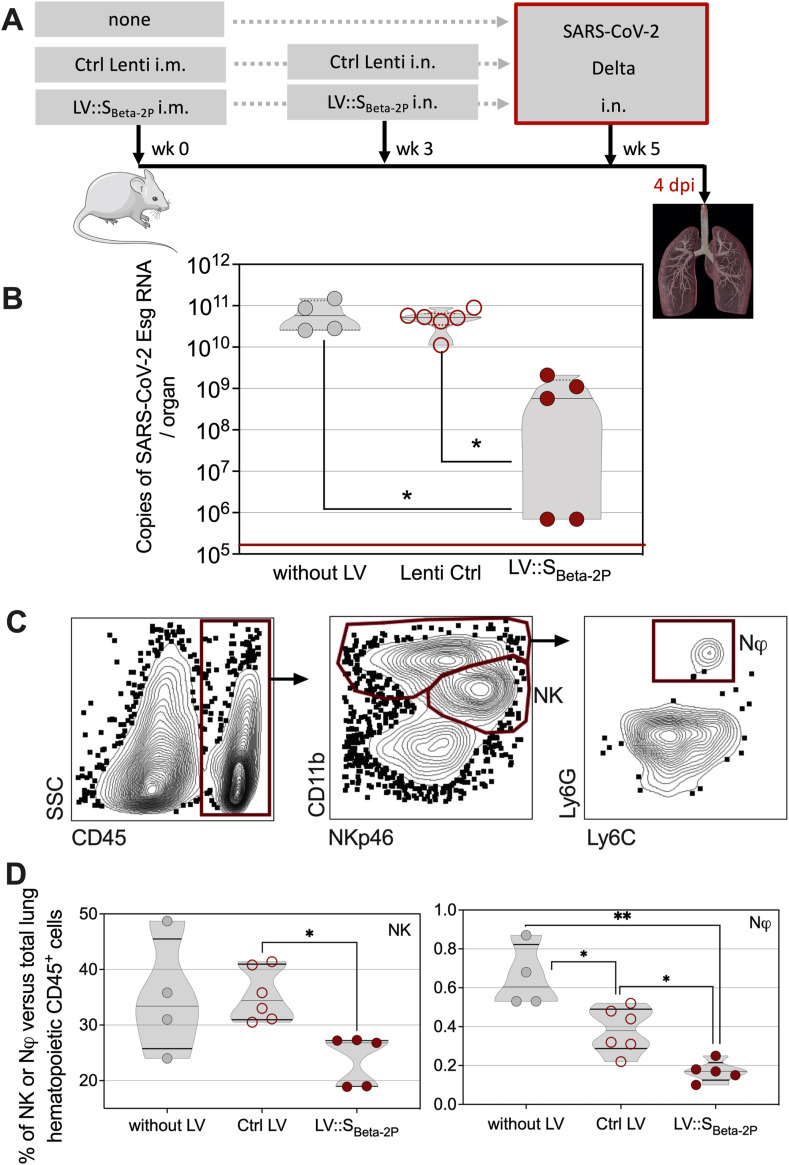
Fig. 5.
Use of HHD-DR1.ACE2Hu1 mice to evaluate the protective capacity of LV::SBeta-2P vaccine candidate against SARS-CoV-2. A. Timeline of vaccination and SARS-CoV-2 challenge. B. HHD-DR1.ACE2Hu1 mice were untreated or primed (i.m.) at wk 0 and boosted (i.n.) at wk 3 with 1 × 108 TU/mouse of LV::SBeta-2P or Ctrl LV, before i.n. challenge at wk 5 with 0.3 × 105 TCID50 of SARS-CoV-2 Delta variant. Lung viral Esg-specific RNA contents was evaluated by qRT-PCR at 4 dpi. Red lines indicate the detection limits. C. Cytometric detection of neutrophils or NK cells in the lungs of untreated, Ctrl-Lenti-, or LV::SBeta-2P-vaccinated and challenged mice at 4 dpi. D. Percentages of neutrophils or NK cells in the lungs of untreated, Ctrl LV-, or LV::SBeta-2P-vaccinated and challenged mice at 4 dpi. Percentages were calculated versus total lung live CD45+ cells. Statistical significance was evaluated by one-way Anova test (*= p < 0.05, **= p < 0.01).