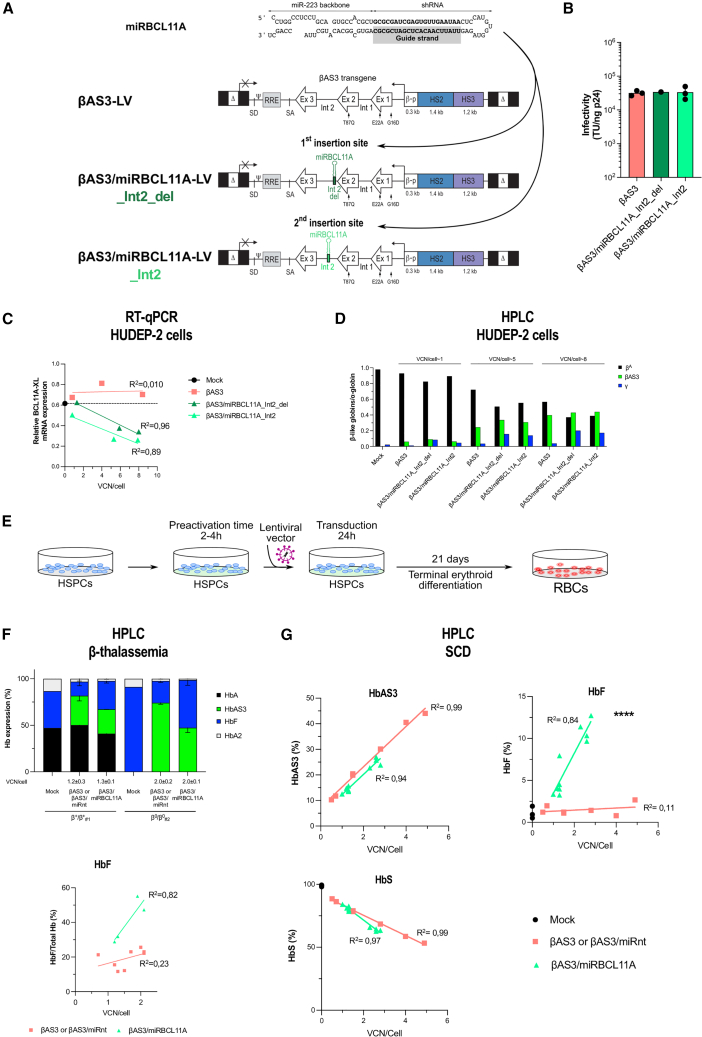
Figure 1.
Bifunctional LVs combining gene addition and gene silencing allow BCL11A-XL silencing and HbF reactivation
(A) The amiRNA (miRBCL11A) is composed of a shRNA embedded in the miR-223 backbone and targets BCL11A-XL mRNA after processing. The guide strand is responsible for recognition of the target mRNA when loaded on the RNA-induced silencing (RISC) complex. To create the novel bifunctional LVs, we inserted miRBCL11A into two distinct sites of intron 2 of the βAS3 transgene (in the βAS3 LV): Int2_del or Int2 (βAS3/miRBCL11A LVs). Δ, deleted HIV-1 U3 region; SD and SA, HIV splicing donor and acceptor sites, respectively; Ψ, HIV-1 packaging signal; RRE, HIV-1 Rev-responsive element; Ex, exons of the human HBB; β-p, promoter of HBB; HS2 and HS3, DNase I hypersensitive site 2 and 3 of human HBB LCR, respectively. Arrows indicate the mutations introduced in exon 1 (generating the G16D and E22A amino acid substitutions) and exon 2 (generating the T87Q amino acid substitution). (B) Infectivity (TU/ng p24) was calculated based on infectious (TUs per milliliter) and physical (p24 antigen ng/mL) titers (Figure S1A) (n = 1–3 independent LV productions for βAS3/miRCL11A_Int2_del and βAS3 and βAS3/miRBCL11A_Int2, respectively). (C) BCL11A-XL expression measured by qRT-PCR in mock- and LV-transduced HUDEP-2 cells during differentiation. mRNA levels were normalized to LMNB2 expression (n = 1 for mock transduced and n = 3 independent biological replicates for LV transduced). BCL11A-XL mRNA expression was not significantly different between cells transduced with the βAS3/miRBCL11A_Int2_del and Int2 LV (linear regression). (D) Globin expression determined by RP HPLC in mock-transduced (n = 1) and LV-transduced cells (n = 3 independent biological replicates per LV). The histogram shows β-like-/α-globin ratios. We observed increased expression of therapeutic γ-globin and similar expression of βAS3-globin in cells transduced with the bifunctional vectors compared with control cells (mock- or βAS3-transduced cells). (E–G) HSPCs from β-thalassemia (F) or SCD (G) donors were either mock transduced or transduced with control (βAS3 or βAS3/miRnt) or bifunctional (βAS3/miRBCL11A) vectors for 24 h. After transduction, cells were differentiated toward the erythroid lineage for 21 days. VCN/cell was measured 14 days after transduction by ddPCR in erythroblasts. Globin expression (HPLC) was evaluated in mature RBCs. βAS3- and βAS3/miRnt-transduced samples were pooled in all analyses (F and G). (F) Hb expression determined by CE HPLC in HSPC-derived RBCs from two β-thalassemia donors (β+/β+#1 and β0/β0#2) after 16 days of differentiation (n = 1–4 independent biological replicates per donor: n = 1 for mock per donor, n = 4 for βAS3 or βAS3/miRnt per donor, and n = 2 βAS3/miRBCL11A per donor). Linear regression of HbF expression vs. VCN/cell confirmed HbF reactivation in RBCs derived from βAS3/miRBCL11A-transduced HSPCs (linear regression, ∗p = 0.0396, n = 4–8 independent biological replicates). (G) HbAS3 (top left panel), HbF (top right panel), and HbS (bottom left panel) expression measured by CE HPLC in HSPC-derived RBCs after 20 days of differentiation (n = 3; two mobilized and one non-mobilized SCD donors). Linear regression for HbF expression, ∗∗∗∗p < 0.0001 (n = 3, 7, and 10 independent biological replicates for mock-, βAS3- or βAS3/miRnt-, and βAS3/miRBCL11A-transduced samples, respectively).