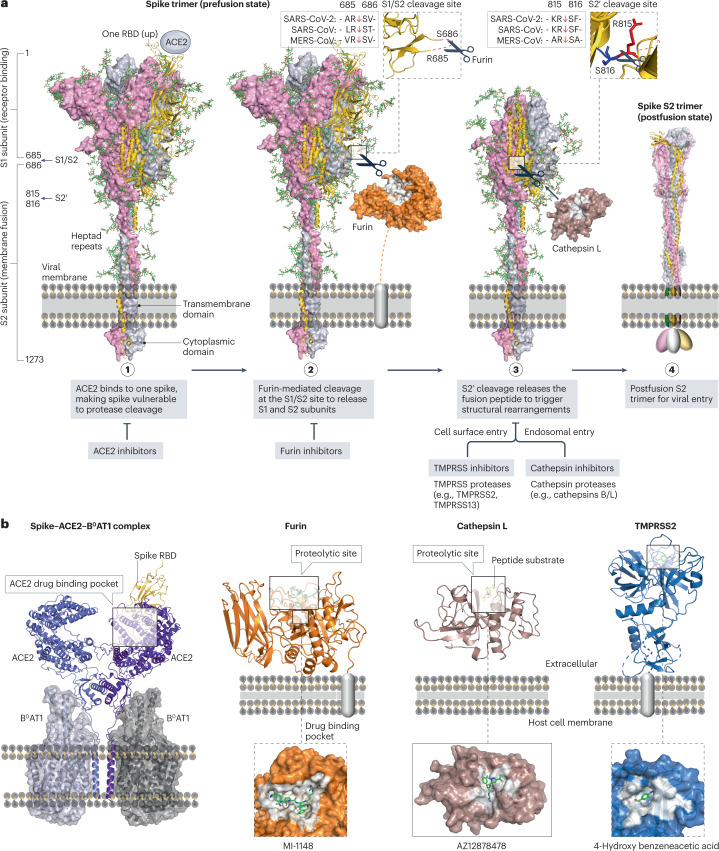
Fig. 7. Interactions between spike, ACE2 and cellular proteases as drug targets.
a, Structural rearrangements of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike trimer from the prefusion state (PDB: 7WEA) to the postfusion state (PDB: 6XRA). Viral entry is initiated via the binding of one prefusion spike with angiotensin-converting enzyme 2 (ACE2), rendering the spike cleavage sites vulnerable to host proteases. The SARS-CoV-2 spike undergoes extensive conformational changes from the closed (down) prefusion state to the open (up) fusion-prone state. Subsequently, furin and cellular proteases (such as cathepsins B/L and transmembrane protease serine subfamily (TMPRSS) 2/13) cleave the S1/S2 site and the S2′ site of the spike, respectively. b, Drug-binding pockets within the spike–ACE2–B0AT1 complex (PDB: 6M17), furin in complex with MI-1148 (PDB: 4RYD), cathepsin L in complex with the Gln-Leu-Ala peptide substrate (PDB: 3K24) and AZ12878478 (PDB: 3HHA), and TMPRSS2 in complex with 4-hydroxy benzeneacetic acid (PDB: 7MEQ), which is the active metabolite of camostat mesylate.