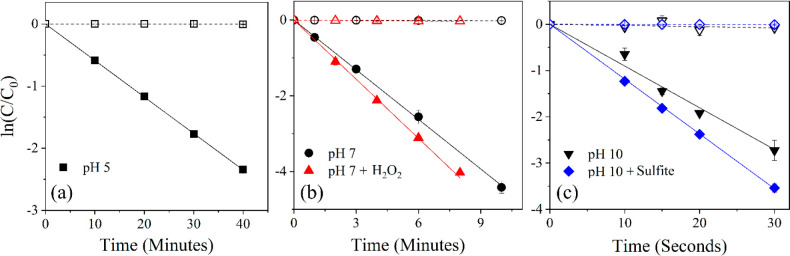
Figure 2.
Photochemical degradation kinetic plots for 2-(trifluoromethyl)phenol (model compound 1a) with photolysis (filled shapes) and dark controls (equivalent hollow shapes) in (a) pH 5 buffer (black squares), (b) pH 7 buffer (black circles) and pH 7 buffer with 1 mM H2O2 (red triangles), and (c) pH 10 buffer (black inverted triangles) and pH 10 buffer with 0.5 mM sulfite (blue diamonds). The photolysis rate constants calculated from the slopes are 3.52 ± 0.07 h–1 for pH 5, 26.4 ± 0.64 h–1 for pH 7, 29.99 ± 1.47 h–1 for H2O2, 334.1 ± 93.45 h–1 for pH 10, and 422.4 ± 9.38 h–1 for sulfite. Error bars represent the standard deviation of triplicate samples. Reported rate constant errors represent the average 95% confidence interval determined by regression statistics. Note the change in scales/units along both the x- and y-axes.