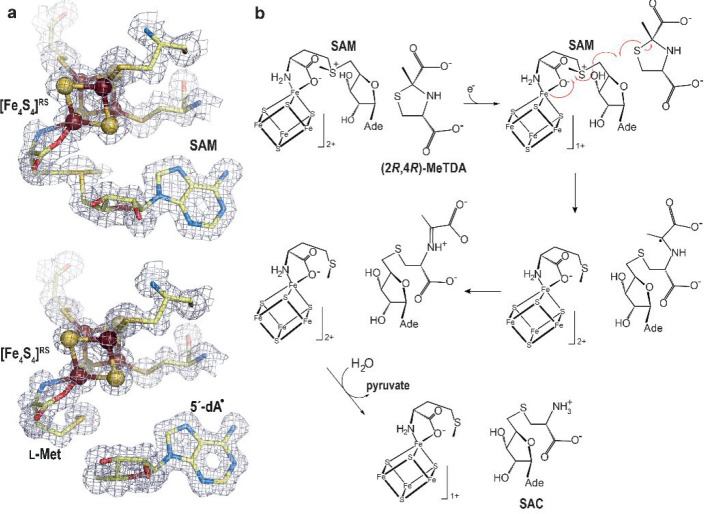
Figure 11.
(a) X-ray model of (top) SAM-bound and (bottom) [5′-dA+Met]-bound TmHydE with the corresponding 2Fo-Fc electron density maps. Map contours were drawn at the 1σ level.68 The [Fe4S4]RS cluster is shown as balls and sticks; SAM or [5′-dA+Met] and cysteine ligands are depicted as sticks. Fe, N, C, O, and S are colored in dark red, blue, light yellow, red, and yellow, respectively. (b) Scheme of the proposed S-adenosyl-l-cysteine (SAC) formation mechanism from (2R,4R)-2-methyl-1,3-thiazolidine-2,4-dicarboxylic acid ((2R,4R)-MeTDA) catalyzed by HydE.66 The SAM C5′–Sγ bond is reductively cleaved with concomitant formation of the C5′–S1 bond and disruption of the (2R,4R)-MeTDA S1–C2 bond. A subsequent one-electron oxidation of the C2-centered radical species into an imine, followed by hydrolysis of the latter would lead to SAC formation. The high-resolution structures and the minimal changes observed during the reaction in crystals allowed us to follow the electronic structure from SAM to the formation of SAC using quantum mechanical/molecular mechanical (QM/MM) calculations.66