Abstract
Psychedelics, also known as classical hallucinogens, affect processes related to perception, cognition and sensory processing mostly via the serotonin 5-HT2A receptor (5-HT2AR). This class of psychoactive substances, which includes lysergic acid diethylamide (LSD), psilocybin, mescaline and the substituted amphetamine 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), is receiving renewed attention for their potential therapeutic properties as it relates to psychiatric conditions such as depression and substance use disorders. Current studies focused on the potentially clinical effects of psychedelics on human subjects tend to exclude sex as a biological variable. Much of the understanding of psychedelic pharmacology is derived from rodent models, but most of this preclinical research has only focused on male mice. Here we tested the effects of DOI on head-twitch behavior (HTR) – a mouse behavioral proxy of human psychedelic potential – in male and female mice. DOI elicited more HTR in female as compared to male C57BL/6J mice, a sex-specific exacerbated behavior that was not observed in 129S6/SvEv animals. Volinanserin (or M100907) – a 5-HT2AR antagonist – fully prevented DOI-induced HTR in male and female C57BL/6J mice. Accumulation of inositol monophosphate (IP1) in the frontal cortex upon DOI administration showed no sex-related effect in C57BL/6J mice. However, the pharmacokinetic properties of DOI differed among sexes – brain and plasma concentrations of DOI were lower 30 and 60 min after drug administration in female as compared to male C57BL/6J mice. Together, these results suggest strain-dependent and sex-related differences in the behavioral and pharmacokinetic profiles of the 5-HT2AR agonist DOI in C57BL/6J mice, and support the importance of studying sex as a biological variable in preclinical psychedelic research.
Keywords: Psychedelics, Classical hallucinogens, Serotonin 5-HT2A receptor, G protein-coupled receptor (GPCR), Sex differences, Head-twitch behavior
1. Introduction
Classical psychedelics, such as lysergic acid diethylamide (LSD), psilocybin, mescaline and 1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane (DOI), have been recognized for their capacity to profoundly dysregulate various mental domains, particularly sensory perception and thought processes [20,44]. Most of the previous studies in rodent models [22,43] and human subjects [9,47] focused their efforts on the effects that occur within minutes to hours after psychedelic administration, with the aim of elucidating the receptor target and signaling pathways involved in their hallucinogenic properties. Importantly, more recent clinical trials suggest that psychedelics may represent a promising novel treatment strategy for patients with depression and other psychiatric conditions. Several studies have reported fast-acting and lasting clinically relevant antidepressant effects upon a single psilocybin administration in combination with psychological support [8,12]. However, the hallucinogenic properties of classical psychedelics preclude their use in daily clinical practice. It is therefore clear that we need a better understanding of the molecular and neural circuit mechanisms underlying the behavioral effects of these drugs with the final goal of developing safer therapeutic alternatives [6,7].
Studies in rodent models and human subjects suggest that most of the acute effects observed directly after psychedelic administration are mediated via activation of the serotonin 5-HT2A receptors (5-HT2ARs), particularly those expressed in forebrain pyramidal neurons [21,32]. Alternative monoaminergic G protein-coupled receptors (GPCRs), such as serotonin 5-HT1A [4], serotonin 5-HT5A [23] and dopamine D2 [40], may also play a role in some of the hallucinogenic properties of this family of psychoactive substances. Nevertheless, the implication of 5-HT2AR-dependent signaling mechanisms in the post-acute effects of psychedelics on preclinical phenotypes that model therapeutically relevant outcomes remains a topic of intense debate. Thus, it has been reported that the effects of DOI or dimethyltryptamine (DMT) on frontal cortex dendritic spine density and acceleration of contextual fear extinction were reduced by the 5-HT2AR antagonist ketanserin or absent in 5-HT2AR knockout mice [17,39]. However, the same 5-HT2AR blocker was unable to fully prevent the post-acute effects of psilocybin on stress-induced hedonic behavior [28] and growth of dendritic spines in the frontal cortex [48]. Some of the most recent studies focused on psychedelic action have included both sexes in their samples [5,41,45,48]. Additionally, it has been reported that the psychedelic 5-HT2AR agonist DOI differentially affects prepulse inhibition of the startle – a model of sensorimotor gating – in male and female 129S6/SvEv mice [52], whereas the 5-HT2AR antagonist volinanserin allosterically augmented the antinociceptive effect of the opioid receptor agonist oxycodone in male but not female C57BL/6J mice [50]. Although these findings suggest sex-related differences in 5-HT2AR-dependent behaviors, the majority of the previous studies focused on the psychedelic properties of 5-HT2AR agonists were carried out exclusively in male rodents [32]. Similarly, while both male and female human participants are generally included in the cohorts to test the effects of psychedelics on behavioral and neuroimaging responses, sex and/or gender as independent experimental variables are usually excluded from the statistical analysis [13,24,53].
This gap in our knowledge related to sex-specific effects of psychedelics is particularly relevant considering that sex and/or gender affects the subjective responses following use of psychoactive substances such as morphine and cocaine [18]. There is also an enhanced vulnerability of women to develop alcohol-related diseases which may be due to sex-related differences in metabolism and gastric tissue activity [2]. Additionally, the subjective peak changes induced by 3,4-methylenedioxy-methamphetamine (MDMA, or ecstasy) were reportedly more intense in women than in men – these included MDMA-induced perceptual changes, thought disturbances, and fear of loss of body control [37].
Head-twitch behavior (HTR) has been demonstrated as a behavioral proxy of human psychedelic potential. This rodent (mouse and rat) behavior – characterized by a side-to-side movement of the head – is exacerbated upon psychedelic administration, and is not induced by other psychoactive drugs such as cocaine, phencyclidine or amphetamine [27,32]. Furthermore, the behavioral potency of different psychedelics on HTR correlates with the psychedelic potency determined in human subjects [25]. Several previous findings based on either pharmacological [26] or gene editing [21,22,33] tools suggest that the 5-HT2AR is the main responsible for the effect of psychedelics on HTR. Consequently, this particular behavior is widely used as a rodent model of classical psychedelic action, yet potential sex-related differences on its manifestation have never been directly evaluated.
Here we characterized the effect of DOI on HTR in two commonly used strains of male and female mice, evaluated in vivo 5-HT2AR-dependent signaling, and tested the pharmacokinetic properties of this phenethylamine psychedelic across sexes. Our data report sex-related differences in the manifestation of DOI-induced HTR as well as changes in DOI distribution across sexes.
2. Materials and methods
2.1. Materials and drugs
For HTR and IP1 assays, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) hydrochloride and (R)-(+)-α-(2,3-dimethox-yphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperinemethanol (volinanser in, also known as M100907) were purchased from Sigma-Aldrich. Drugs were dissolved in saline (0.9 % NaCl) to the appropriate volume (5 μl/g body weight), and administered intraperitonally (i.p.). Vehicle-treated condition denotes injection (i.p.) of saline (0.9 %) to the equivalent volume of the drug administered. For pharmacokinetic studies, analytical references DOI and (±)-2,5-dimethoxy-4-bromophenethylamine (2-CB) were purchased from Cerilliant Corporation. Acetonitrile, ammonium acetate, ethyl acetate, formic acid, hexane, methanol, sodium hydroxide and water were purchased from Fisher Scientific. All other chemicals were obtained from standard sources.
2.2. Animals
All assays were performed on adult (8 – 14 week-old) male and female C57BL6/J (Jackson Laboratories) and/or 129S6/SvEv (Taconic Biosciences) mice. For HTR assays, animals were bred in-house following previous purchase of breeding trios (C57BL6/J mice from Jackson Laboratories, and 129S6/SvEv from Taconic Biosciences). All HTR experiments were carried out in adult (8 – 12 week-old) male and female littermates. Pharmacokinetic and IP1 assays were performed on adult (pharmacokinetics, 8 week-old; IP1, 10 – 14 week-old) C57BL6/J male and female mice received from Jackson Laboratories and acclimated to the vivarium for two weeks. Sex classification of all mice was based on anogenital distance [30]. Animals were housed (2 – 5 mice per cage) on a 12 h light/dark cycle (lights on 6 a.m. to 6 p.m.) at 23 °C with food and water ad libitum, except during behavioral testing. All experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines, and were approved by the Virginia Commonwealth University Animal Care and Use Committee. Behavioral testing took place between 9 a.m. to 6 p.m. All efforts were made to minimize animal suffering and the number of animals used.
2.3. Head-twitch response (HTR)
HTR assays using magnetic ear-tagging were performed as previously reported [15,16]. Our previous findings also indicated a high correlation between the automated detection of HTR and the visually detected videotaped HTRs [15,16]. Testing occurred no more than once per week with at least 7 days between test sessions. On test days, mice were placed individually into the coiled chamber for 30 min to acclimate to the environment and determine baseline HTR. Subsequently, DOI (0.5 or 2 mg/kg) or vehicle was administered immediately prior to HTR recording, whereas volinanserin (0.032 mg/kg) or vehicle was administered 15 min prior to DOI. Doses of DOI and volinanserin were selected based on prior HTR studies [14,33,50,52].
2.4. IP1 accumulation
IP1 accumulation assays in mouse frontal cortex samples were performed as previously reported [14]. Briefly, mice were sacrificed by cervical dislocation 60 min after DOI (2 mg/kg) or vehicle administration, and bilateral frontal cortex (bregma 1.90 to 1.40 mm) was dissected and immediately frozen at − 80 °C until analysis.
2.5. DOI distribution
Blood and brain tissue samples were obtained from male and female mice following DOI (2 mg/kg) administration. Mice were decapitated 30 min, 1 h and 4 h after drug administration; controls were mock-injected mice. Ultra-high-performance liquid chromatograph tandem mass spectrometer (UHPLC-MS/MS) assays in blood and frontal cortex samples were performed as previously reported [15,49,50].
2.6. Statistical analysis
Statistical significance of experiments involving two or more groups and two or more treatments was assessed by two-way (or three-way) ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Bonferroni’s post hoc test. One-way and two-way ANOVA analysis was performed with GraphPad Prism software version 9. Three-way ANOVA analysis was performed in R v.4.1.0 environment using the aov function. The level of significance was set at P = 0.05. All values in the figure legends represent mean ± S.E.M.
3. Results
3.1. DOI-induced HTR in male and female C57BL/6J mice
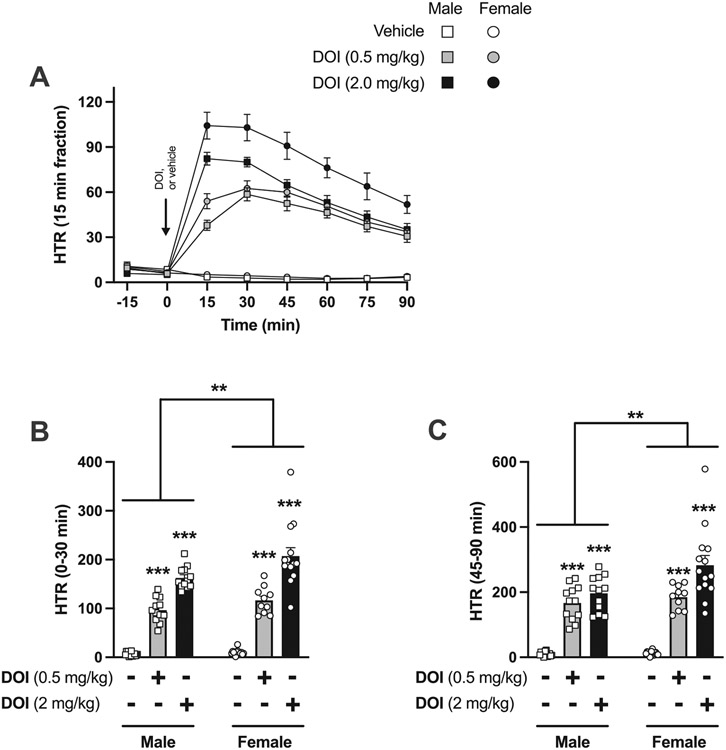
We first tested the effect of the psychedelic DOI on HTR in male and female C57BL/6J mice. Low (0.5 mg/kg) and moderate (2 mg/kg) doses of DOI were chosen based on previous findings with this classical psychedelic [33,52]. In time-course assays (Fig. 1A), three-way ANOVA analysis (0–90 min) revealed significant main effects of time, sex, and dose, as well as a significant interaction between time and dose, and sex and dose (Table 1).
Fig. 1.
Effect of DOI on HTR in male (n = 12–15) and female (n = 10–15) C57BL/6J mice. A) Time-course showing HTR counts in 15 min blocks corresponding to different doses of DOI (0.5 mg/kg and 2 mg/kg) or vehicle. Back arrow shows the administration time-point (t = 0). B) Sum of HTR during the first 30 min after DOI or vehicle administration. C) Sum of HTR during 45–90 min after DOI or vehicle administration. Two-way ANOVA followed by Bonferroni’s post hoc test (**P < 0.01, ***P < 0.001). For a three-way ANOVA analysis, see Table 1. Data show mean ± S.E.M.
Table 1.
Three-way ANOVA analysis of the effect of DOI on HTR (0–90 min) in male and female C57BL/6J mice (see also Fig. 1A).
| Source of variation | ANOVA | p value |
|---|---|---|
| Time | F[6,469] = 77.66 | p < 0.001 |
| Sex | F[1,469] = 109.66 | p < 0.001 |
| Dose | F[2,469] = 631.74 | p < 0.001 |
| Time × Sex | F[6,469] = 4.10 | n.s. |
| Time × Dose | F[12,469] = 28.74 | p < 0.001 |
| Sex × Dose | F[2,469] = 17.22 | p < 0.001 |
| Time × Sex × Dose | F[12,469] = 0.51 | n.s. |
n.s., not significant.
This effect of time and treatment with either dose of DOI on HTR was also evident when both sexes were analyzed separately (0–90 min) (Fig. 1A) (two-way ANOVA – 0.5 mg/kg DOI vs vehicle in males: treatment F[1,175] = 735.3, P < 0.001; time F[6,175] = 20.25, P < 0.001; interaction F[6,175] = 32.25, P < 0.001) (two-way ANOVA – 2.0 mg/kg DOI vs vehicle in males: treatment F[1,175] = 1430, P < 0.001; time F[6,175] = 56.80, P < 0.001; interaction F[6,175] = 72.15, P < 0.001) (two-way ANOVA – 0.5 mg/kg DOI vs vehicle in females: treatment F[1,161] = 1074, P < 0.001; time F[6,161] = 34.36, P < 0.001; interaction F[6,161] = 39.20, P < 0.001) (two-way ANOVA – 2.0 mg/kg DOI vs vehicle in females: treatment F[1,189] = 576.1, P < 0.001; time F [6,189] = 20.97, P < 0.001; interaction F[6,189] = 22.15, P < 0.001).
No differences across sexes in response to vehicle administration were observed (0–90 min) (Fig. 1A) (two-way ANOVA – male vs female in vehicle mice: sex F[1,224] = 0.19, P > 0.05; time F[7,224] = 13.66, P < 0.001; interaction F[7,224] = 1.14, P > 0.05).
Additionally, the difference in HTR among sexes was corroborated on the collapsed HTR counts over the peak effect during the first 30 min following DOI (or vehicle) administration (Fig. 1B) (one-way ANOVA – DOI effect in males: F[2,36] = 237.9, P < 0.001) (one-way ANOVA – DOI effect in females: F[2,36] = 82.48, P < 0.001) (two-way ANOVA – DOI effect in both sexes: treatment F[2,72] = 211.9, P < 0.001; sex F[1,72] = 9.39, P < 0.01; interaction F[2,72] = 2.92, P = 0.06), as well as during 45–90 min after drug or vehicle administration (Fig. 1C) (one-way ANOVA – DOI effect in males: F[2,36] = 78.25, P < 0.001) (one-way ANOVA – DOI effect in females: F[2,36] = 55.42, P < 0.001) (two-way ANOVA – DOI effect in both sexes: treatment F[2,72] = 116.9, P < 0.001; sex F[1,72] = 7.29, P < 0.01; interaction F[2,72] = 3.96, P < 0.05),
To assess the role of the 5-HT2AR in HTR across sexes, a potent and relatively selective 5-HT2AR antagonist – volinanserin (0.032 mg/kg) [10] – was administered 15 min prior to DOI (2 mg/kg). This dose of volinanserin was selected based on previous findings [33]. In time-course assays (Fig. 2A), three-way ANOVA analysis (0–90 min) revealed significant main effects of time, sex, and treatment, as well as a significant interaction between time and treatment, and sex and treatment (Table 2).
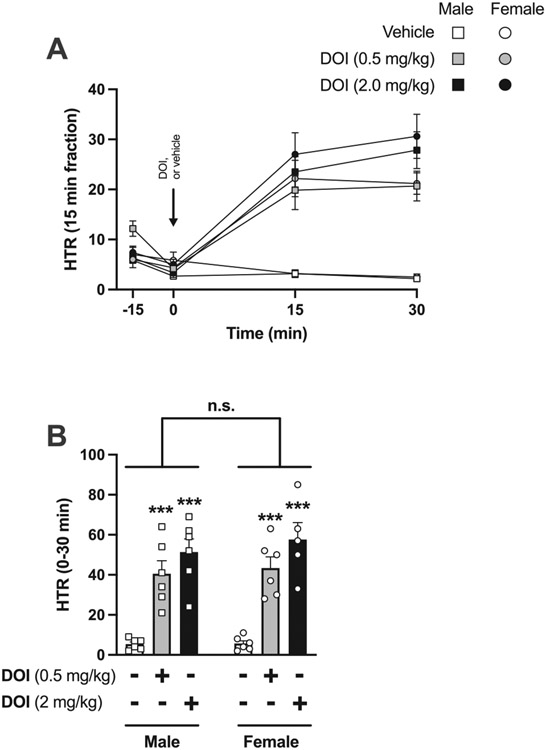
Fig. 2.
Effect of volinanserin (vol) on DOI-induced HTR in male (n = 6) and female (n = 6) C57BL/6J mice. A) Time-course showing HTR counts in 15 min blocks corresponding to mice injected with DOI (2 mg/kg) or vehicle 15 min after being injected with volinanserin (0.032 mg/kg) or vehicle. Back arrows show the administration time-point of volinanserin/vehicle (t = −15) and DOI/vehicle (t = 0). B) Sum of HTR during the first 30 min after DOI or vehicle administration. C) Sum of HTR during 45–90 min after DOI or vehicle administration. Two-way ANOVA followed by Bonferroni’s post hoc test (*P < 0.05, **P < 0.01, ***P < 0.001, n. s., not significant). For a three-way ANOVA analysis, see Table 2. Data show mean ± S.E.M.
Table 2.
Three-way ANOVA analysis of the effect of volinanserin on DOI-induced HTR (0–90 min) in male and female C57BL/6J mice (see also Fig 2A).
| Source of variation | ANOVA | p value |
|---|---|---|
| Time | F[6,210] = 19.13 | p < 0.001 |
| Sex | F[1,210] = 46.77 | p < 0.001 |
| Treatment | F[2,210] = 414.93 | p < 0.001 |
| Time × Sex | F[6,210] = 1.32 | n.s. |
| Time × Treatment | F[12,210] = 22.29 | p < 0.001 |
| Sex × Treatment | F[2,210] = 31.99 | p < 0.001 |
| Time × Sex × Treatment | F[12,210] = 1.06 | n.s. |
n.s., not significant.
This effect of time and DOI/volinanserin treatment on HTR in both sexes was also evident when both sexes were analyzed separately (0–90 min) (Fig. 2A) (two-way ANOVA – DOI and/or volinanserin vs vehicle in males: treatment F[2,105] = 407.3, P < 0.001; time F[6,105] = 23.93, P < 0.001; interaction F[12,105] = 29.26, P < 0.001) (two-way ANOVA – DOI and/or volinanserin vs vehicle in females: treatment F[2,105] = 195.3, P < 0.001; time F[6,105] = 8.13, P < 0.001; interaction F[12,105] = 8.98, P < 0.001). Post hoc analysis also showed a difference between DOI and vehicle, but not between volinanserin + DOI and vehicle (males: DOI vs vehicle, P < 0.001; volinanserin + DOI vs vehicle, P > 0.05) (females: DOI vs vehicle, P < 0.001; volinanserin + DOI vs vehicle, P > 0.05).
As before (see Fig. 1A, above), no differences across sexes in response to vehicle administration were observed (0–90 min) (Fig. 2A) (two-way ANOVA – male vs female in vehicle mice: sex F[1,90] = 0.24, P > 0.05; time F[8,90] = 7.74, P < 0.001; interaction F[8,90] = 0.22, P > 0.05).
Additionally, as above (see Fig. 1B and Fig. 1C), differences across sexes were observed with the sum of HTR during the first 30 min following administration of DOI and/or volinanserin (or vehicle) (Fig. 2B) (one-way ANOVA – DOI and/or volinanserin effect in males: F[2,15] = 379.5, P < 0.001) (one-way ANOVA – DOI and/or volinanserin effect in females: F[2,15] = 52.23, P < 0.001) (two-way ANOVA – DOI and/or volinanserin effect in both sexes: treatment F[2,30] = 139.7, P < 0.001; sex F[1,30] = 5.83, P < 0.05; interaction F[2,30] = 5.45, P < 0.01), as well as during 45–90 min post drug or vehicle administration (Fig. 2C) (one-way ANOVA – DOI and/or volinanserin effect in males: F[2,15] = 37.43, P < 0.001) (one-way ANOVA – DOI and/or volinanserin effect in females: F[2,15] = 26.45, P < 0.001) (two-way ANOVA – DOI and/or volinanserin effect in both sexes: treatment F[2,30] = 50.21, P < 0.001; sex F[1,30] = 9.78, P < 0.01; interaction F[2,30] = 5.88, P < 0.01).
3.2. DOI-induced HTR in male and female 129S6/SvEv mice
To evaluate potential strain-dependent variations, we tested the effect of DOI on HTR in male and female 129S6/SvEv mice. Based on our previous HTR findings with this particular strain of mice [52], as well previous publications by other groups showing reduced locomotor activity in 129S6/SvEv mice as compared to other strains, including C57BL/6J animals [38], we assessed time-course HTR assays during the first 30 min after DOI (0.5 or 2.0 mg/kg) or vehicle administration. As above with C57BL/6J mice (see Table 1), three-way ANOVA analysis (0–30 min) showed significant main effects of time and dose, as well as a significant interaction between time and dose (Fig. 3A and Table 3). However, three-way ANOVA analysis (0–30 min) also showed absence of sex-related effects on DOI-induced HTR in 129S6/SvEv mice (Fig. 3A and Table 3).
Fig. 3.
Effect of DOI on HTR in male (n = 6) and female (n = 5–6) 129S6/SvEv mice. A) Time-course showing HTR counts in 15 min blocks corresponding to different doses of DOI (0.5 mg/kg and 2 mg/kg) or vehicle. Back arrow shows the administration time-point (t = 0). B) Sum of HTR during the first 30 min after DOI or vehicle administration. Two-way ANOVA followed by Bonferroni’s post hoc test (***P < 0.001, n.s., not significant). For a three-way ANOVA analysis, see Table 3. Data show mean ± S.E.M.
Table 3.
Three-way ANOVA analysis of the effect of DOI on HTR (0–30 min) in male and female 129S6/SvEv mice (see also Fig. 3A).
| Source of variation | ANOVA | p value |
|---|---|---|
| Time | F[2,87] = 53.84 | p < 0.001 |
| Sex | F[1,87] = 1.07 | n.s. |
| Dose | F[2,87] = 74.07 | p < 0.001 |
| Time × Sex | F[2,87] = 0.10 | n.s. |
| Time × Dose | F[4,87] = 19.22 | p < 0.001 |
| Sex × Dose | F[2,87] = 0.20 | n.s. |
| Time × Sex × Dose | F[4,87] = 0.21 | n.s. |
n.s., not significant.
This effect of time and treatment with either dose of DOI on HTR in both sexes was also evident when both sexes were analyzed separately (0–30 min) (Fig. 3A) (two-way ANOVA – 0.5 mg/kg DOI vs vehicle in males: treatment F[1,30] = 52.93, P < 0.001; time F[2,30] = 10.18, P < 0.001; interaction F[2,30] = 10.28, P < 0.001) (two-way ANOVA – 2.0 mg/kg DOI vs vehicle in males: treatment F[1,30] = 87.74, P < 0.001; time F[2,30] = 20.43, P < 0.001; interaction F[2,30] = 20.95, P < 0.001) (two-way ANOVA – 0.5 mg/kg DOI vs vehicle in females: treatment F[1,30] = 58.55, P < 0.001; time F[2,30] = 9.29, P < 0.001; interaction F[2,30] = 18.51, P < 0.001) (two-way ANOVA – 2.0 mg/kg DOI vs vehicle in females: treatment F[1,27] = 71.50, P < 0.001; time F[2,27] = 12.02, P < 0.001; interaction F[2,27] = 20.04, P < 0.001).
No differences across sexes in response to vehicle administration were observed (Fig. 3A) (two-way ANOVA – male vs female in vehicle mice: sex F[1,40] = 2.24, P > 0.05; time F[3,40] = 5.15, P < 0.01; interaction F[3,40] = 0.83, P > 0.05).
The absence of sex-related differences in 129S6/SvEv mice was corroborated on the collapsed HTR counts over the peak effect during the first 30 min following DOI (or vehicle) administration (Fig. 3C) (one-way ANOVA – DOI effect in males: F[2,15] = 19.67, P < 0.001) (one-way ANOVA – DOI effect in females: F[2,14] = 23.12, P < 0.001) (two-way ANOVA – DOI effect in both sexes: treatment F[2,29] = 42.75, P < 0.001; sex F[1,29] = 0.49, P > 0.05; interaction F[2,29] = 0.14, P > 0.05).
3.3. Spontaneous HTR in both sexes of C57BL/6J and 129S6/SvEv mice
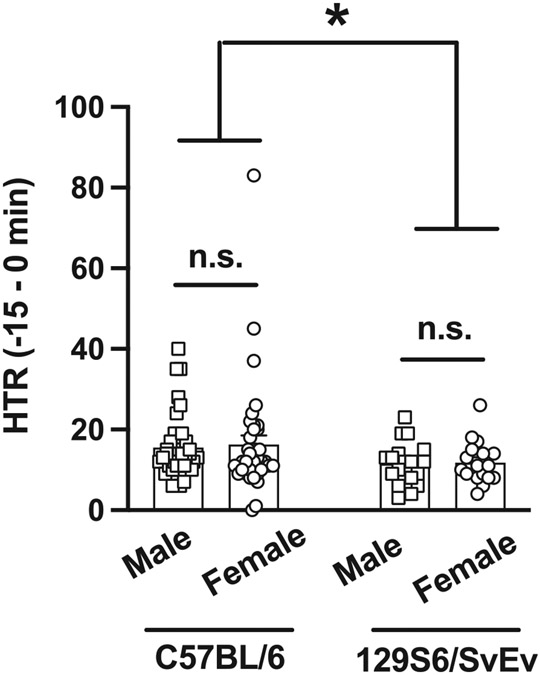
We next explored potential changes in spontaneous HTR (i.e., HTR in the absence of psychedelic administration) in both sexes of C57BL/6J and 129S6/SvEv mice (Fig. 4). There was an absence of sex differences on DOI-induced HTR in either C57BL/6J or 129S6/SvEv mice. However, spontaneous HTR was significantly reduced in 129S6/SvEv as compared to C57BL/6J male and female animals (two-way ANOVA: sex F[1,108] = 0.07, P > 0.05; strain F[1,108] = 4.52, P < 0.05; interaction F[1,108] = 0.01, P > 0.05).
Fig. 4.
Spontaneous HTR in both sexes of C57BL/6J (n = 38–39) and 129S6/SvEv (17–18) mice. A) Total HTR counts during the 15 min before DOI or vehicle administration (see also Fig. 1 and Fig. 3). Two-way ANOVA followed by Bonferroni’s post hoc test (*P < 0.05, n.s., not significant). Data show mean ± S.E.M.
3.4. DOI-induced IP1 accumulation in the frontal cortex of male and female C57BL/6J mice
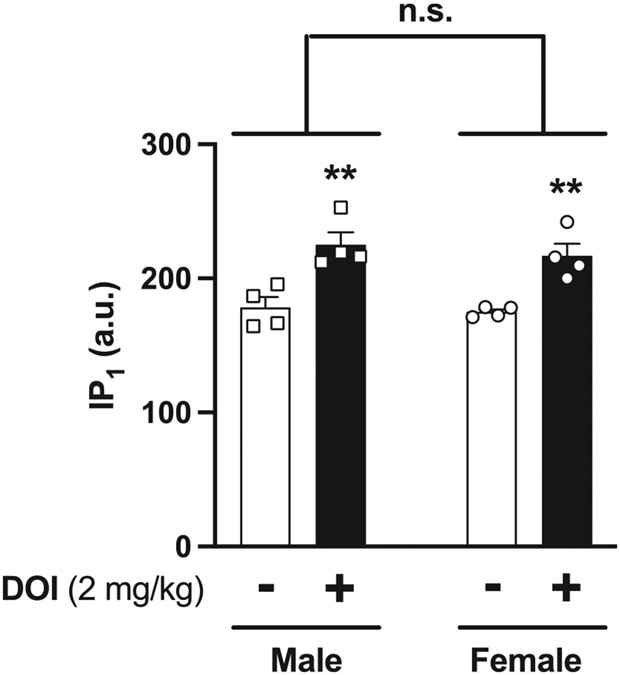
We previously reported that the density of the 5-HT2AR was reduced in the frontal cortex of female C57BL/6J mice as compared to male littermates. This was assessed by [3H]ketanserin binding assays in membrane preparations of frontal cortex samples [50]. Here we found a similar trend in a separate cohort of male and female C57BL/6J mice (data not shown). To further evaluate potential sex differences related to 5-HT2AR-dependent signaling, we tested the accumulation of IP1, a downstream effector of the Gq/11 signaling pathway in vivo, in the frontal cortex of male and female C57BL/6J mice. As expected based on our previous finding using this functional assay with alternative 5-HT2AR agonists [14], DOI-treated animals showed greater accumulation of IP1 in the frontal cortex. However, no sex-related differences were observed (Fig. 5) (two-way ANOVA: DOI effect F[1,12] = 33.99, P < 0.001; sex F[1,12] = 0.59, P > 0.05; interaction F[1,12] = 0.10, P > 0.05).
Fig. 5.
Effect of DOI on IP1 accumulation in the frontal cortex of male (n = 4) and female (n = 4) C57BL/6J mice. A) Samples were collected 60 min after DOI (2 mg/kg) or vehicle administration. Two-way ANOVA followed by Bonferroni’s post hoc test (**P < 0.01, n.s., not significant). Data show mean ± S.E.M.
3.5. Distribution of DOI in blood and frontal cortex of male and female C57BL/6J mice
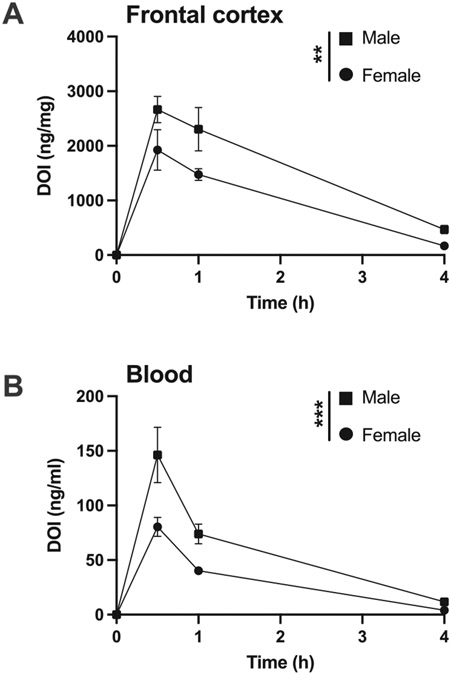
We evaluated whether these sex differences on DOI-induced HTR were related to pharmacokinetic variations between male and female mice by testing the distribution of DOI in plasma and the CNS. Mice were administered DOI (2 mg/kg) and blood and frontal cortex samples were collected at different time-points after drug administration. Control group (t = 0) were mock-injected mice. As previously reported in male C57BL/6J mice [15], concentrations of DOI were higher in brain samples as compared to blood (Fig. 6). Similarly, the absorption of DOI was fast, and reached its maximal concentration during the first ~ 30 min in frontal cortex (Fig. 6A) and blood (Fig. 6B) tissue samples in both male and female mice. Interestingly, concentrations of DOI were higher in brain (Fig. 6A) (two-way ANOVA: sex F[1,22] = 10.00, P < 0.01; time F[3,22] = 58.97, P < 0.001; interaction F[3,22] = 1.73, P > 0.05) and blood (Fig. 6B) two-way ANOVA: sex F[1,24] = 14.34, P < 0.001; time F[3,24] = 54.61, P < 0.001; interaction F[3,24] = 4.41, P < 0.05) samples of male as compared to female C57BL/6J mice.
Fig. 6.
Brain and blood concentrations of DOI after a single administration (2 mg/kg) in male (n = 4) and female (n = 3–4) C57BL/6J mice. A) Frontal cortex concentrations of DOI 0.5, 1, and 4 h after a single administration. B) Blood concentrations of DOI 0.5, 1, and 4 h after a single administration. Two-way ANOVA followed by Bonferroni’s post hoc test (*P < 0.01, ***P < 0.001). Data show mean ± S.E.M.
4. Discussion
Most of the previous studies on the effect of classical psychedelics on mouse HTR did not include sex as an experimental variable. To the best of our knowledge, our findings represent the first evidence of strain-dependent and sex-related differences in the behavioral response to psychedelics in mice.
Using two separate doses of the phenethylamine psychedelic DOI, we show that the number of HTRs was more abundant in female as compared to male C57BL/6J mice. This sex-dependent effect was not observed in male and female 129S6/SvEv mice. Previous findings convincingly demonstrate that the 5-HT2AR antagonist volinanserin eliminates DOI-induced HTR [16,33], but these studies were carried out in male mice only. Here we validate that volinanserin also fully prevents HTR induced at two individual doses of the psychedelic DOI in female C57BL/6J mice. Accumulation of IP1 in the frontal cortex as a functional readout of 5-HT2AR activation upon DOI administration was comparable among sexes, whereas a sex-specific effect was observed for the pharmacokinetic properties of DOI in blood and brain samples, where concentrations of DOI were lower in female as compared to male C57BL/6J mice.
Although psychedelics pronouncedly exacerbate HTR, this side-to-side movement of the head is also observed as a spontaneous behavior in the absence of psychedelic administration [27]. Here we report that the frequency of spontaneous HTR (tested during the time-course assays before DOI or vehicle administration) was similar between sexes in both C57BL/6J and 129S6/SvEv mice. This suggests that the sex effect on DOI-induced HTR is contingent on strain-specific differences. Similarly, it has been previously suggested [15,16,21,31,33,42,52], but not formally proven, that spontaneous HTR is lower in 129S6/SvEv as compared to strains such as C57BL/6J and CD1. Our findings show a significant sex-independent reduction in the counts of spontaneous HTR in 129S6/SvEv as compared to C57BL/6J mice. We also corroborate that DOI-induced HTR is reduced across sexes in 129S6/SvEv mice versus C57BL/6J animals. Similar findings have been reported related to ambulatory motor activity, with large strain and robust sex effects, as well as higher rearing and locomotor activity in C57BL/6 female mice [35]. Our findings also correlate with previous studies suggesting that 129S6/SvEv mice present lower locomotor, rearing and exploratory activity [36]. It will be interesting explore the effects of sex and strain on the outcomes of DOI and other structural classes of psychedelics with alternative phenotypes that model a potential therapeutic action such as reduction of immobility time in the forced-swim test, acceleration of fear extinction, and lasting alterations in dendritic spine structure and chromatin organization [17]. Furthermore, the extent and magnitude of sex and strain differences in the response to psychedelics could be assessed in genetically diverse outbred rodents to explore potential genotype-phenotype relationships [19].
As mentioned above, we previously reported that the density of 5-HT2AR is reduced in the frontal cortex of female mice, as compared to male C57BL/6J littermates [50]. Pharmacokinetic sex-related differences may potentially be linked to compensatory mechanisms that underlie at least some of the differences in DOI-induced HTR between male and female C57BL/6J mice. However, DOI-accumulation of IP1 in the frontal cortex was comparable between both sexes. Although the potential role of this 5-HT2AR-dependent signaling pathway in HTR remains unexplored, a potential explanation may be related to the presence of receptor reserve by which orthosteric agonists need to activate only a small fraction of the existing receptor population to produce the maximal response [34].
Stress and other environmental factors affect DOI-induced HTR in mice [29]. To minimize the influence of environmental stressors on our experimental outcomes, animals tested in the HTR assays were littermates - these included both sexes of C57BL/6J and 129S6/SvEv mice. Further assays are needed to evaluate whether environmental factors such as maternal behavior or shipment differentially affect sex-specific 5-HT2AR-dependent processes such as frontocortical IP1 accumulation and distribution of DOI. Similarly, additional investigation will test for interactions with estrous cycle status [54], as well as the role of ovarian hormones [3], estradiol [51] and BDNF [11] in regulating 5-HT2AR expression in female rodents.
The phenomenon of female preponderance in depression has been well-reported across different cultures. However, this concept is also challenged by higher rates of suicide and addictive behaviors in males, and a longer life-span in females [1,46]. Considering the therapeutic potential of psychedelics in several psychiatric disorders [8,12,32], our findings highlight the need of more comprehensive preclinical and clinical study designs that evaluate effects of psychedelics across sexes with the goal of developing personalized approaches to help determine the optimum dose and timing of psychedelic therapies.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grants R01MH084894 (J.G.-M.), R01MH111940 (J.G.-M.), P30DA033934 (J.L.P), and T32MH020030 (J.Y. and M.d.l.F.R.).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Abate KH, Gender disparity in prevalence of depression among patient population: a systematic review, Ethiop J. Health Sci 23 (2013) 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Ghayes ZW, Schaefer C, Lieber CS, Gender differences in pharmacokinetics of alcohol, Alcohol. Clin. Exp. Res 25 (2001) 502. [PubMed] [Google Scholar]
- [3].Birzniece V, Johansson IM, Wang MD, Backstrom T, Olsson T, Ovarian hormone effects on 5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptor mRNA expression in the ventral hippocampus and frontal cortex of female rats, Neurosci. Lett 319 (2002) 157. [DOI] [PubMed] [Google Scholar]
- [4].Callahan PM, Appel JB, Differentiation between the stimulus effects of (+)-lysergic acid diethylamide and lisuride using a three-choice, drug discrimination procedure, Psychopharmacology 100 (1990) 13. [DOI] [PubMed] [Google Scholar]
- [5].Cameron LP, Benson CJ, DeFelice BC, Fiehn O, Olson DE, Chronic, Intermittent Microdoses of the Psychedelic N, N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents, ACS Chem. Neurosci 10 (2019)3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, Rabow ZT, Fiehn O, Wulff H, McCorvy JD, Lein PJ, Kokel D, Ron D, Peters J, Zuo Y, Olson DE, A non-hallucinogenic psychedelic analogue with therapeutic potential, Nature 589 (2021) 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cao D, Yu J, Wang H, Luo Z, Liu X, He L, Qi J, Fan L, Tang L, Chen Z, Li J, Cheng J, Wang S, Structure-based discovery of nonhallucinogenic psychedelic analogs, Science 375 (2022) 403. [DOI] [PubMed] [Google Scholar]
- [8].Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, Nutt DJ, Trial of Psilocybin versus Escitalopram for Depression, N. Engl. J. Med 384 (2021) 1402. [DOI] [PubMed] [Google Scholar]
- [9].Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX, Psilocybin impairs high-level but not low-level motion perception, NeuroReport 15 (2004) 1947. [DOI] [PubMed] [Google Scholar]
- [10].Casey AB, Cui M, Booth RG, Canal CE, “Selective” serotonin 5-HT2A receptor antagonists, Biochem. Pharmacol 200 (2022), 115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chhibber A, Zhao L, ERbeta and ApoE isoforms interact to regulate BDNF-5-HT2A signaling and synaptic function in the female brain, Alzheimers Res. Ther 9 (2017) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, Griffiths RR, Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial, JAMA Psychiatry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, Roseman L, Nutt D, Carhart-Harris R, Increased global integration in the brain after psilocybin therapy for depression, Nat. Med 28 (2022) 844. [DOI] [PubMed] [Google Scholar]
- [14].de la Fuente Revenga M, Shah UH, Nassehi N, Jaster AM, Hemanth P, Sierra S, Dukat M, Gonzalez-Maeso J, Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice, ACS Chem. Neurosci (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de la Fuente Revenga M, Shin JM, Vohra HZ, Hideshima KS, Schneck M, Poklis JL, Gonzalez-Maeso J, Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo, Sci. Rep 9 (2019) 14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de la Fuente Revenga M, Vohra HZ, Gonzalez-Maeso J, Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection, J. Neurosci. Methods 334 (2020), 108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].de la Fuente Revenga M, Zhu B, Guevara GA, Naler LB, Saunders JM, Zhou Z, Toneatti R, Sierra S, Wolstenholme J, Beardsley PM, Huntley GW, Lu C, Gonzalez-Maeso J, Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice, Cell Rep 37 (2021), 109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fattore L, Marti M, Mostallino R, Castelli MP, Sex and Gender Differences in the Effects of Novel Psychoactive Substances, Brain Sci. 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gileta AF, Fitzpatrick CJ, Chitre AS, St Pierre CL, Joyce EV, Maguire RJ, McLeod AM, Gonzales NM, Williams AE, Morrow JD, Robinson TE, Flagel SB, Palmer AA, Genetic characterization of outbred Sprague Dawley rats and utility for genome-wide association studies, PLoS Genet. 18 (2022) e1010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Glennon RA, Classical hallucinogens: an introductory overview, NIDA Res. Monogr 146 (1994) 4. [PubMed] [Google Scholar]
- [21].Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA, Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior, Neuron 53 (2007) 439. [DOI] [PubMed] [Google Scholar]
- [22].Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC, Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex, J. Neurosci 23 (2003) 8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R, Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor, Neuron 22 (1999) 581. [DOI] [PubMed] [Google Scholar]
- [24].Griffiths R, Richards W, Johnson M, McCann U, Jesse R, Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later, J. Psychopharmacol 22 (2008) 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD, Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species, Neuropharmacology 167 (2020), 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Halberstadt AL, Geyer MA, Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement, Psychopharmacology 227 (2013) 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hanks JB, Gonzalez-Maeso J, Animal models of serotonergic psychedelics, ACS Chem. Neurosci 4 (2013) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM, Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice, Proc. Natl. Acad. Sci. U. S. A 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, Gonzalez-Maeso J, Prenatal Stress Induces Schizophrenia-Like Alterations of Serotonin 2A and Metabotropic Glutamate 2 Receptors in the Adult Offspring: Role of Maternal Immune System, J. Neurosci 33 (2013) 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hotchkiss AK, Vandenbergh JG, The anogenital distance index of mice (Mus musculus domesticus): an analysis, Contemp. Top. Lab. Anim. Sci 44 (2005) 46. [PubMed] [Google Scholar]
- [31].Ibi D, de la Fuente Revenga M, Kezunovic N, Muguruza C, Saunders JM, Gaitonde SA, Moreno JL, Ijaz MK, Santosh V, Kozlenkov A, Holloway T, Seto J, Garcia-Bea A, Kurita M, Mosley GE, Jiang Y, Christoffel DJ, Callado LF, Russo SJ, Dracheva S, Lopez-Gimenez JF, Ge Y, Escalante CR, Meana JJ, Akbarian S, Huntley GW, Gonzalez-Maeso J, Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects, Nat. Neurosci 20 (2017) 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jaster AM, de la Fuente Revenga M, Gonzalez-Maeso J, Molecular targets of psychedelic induced plasticity, J. Neurochem (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jaster AM, Elder H, Marsh SA, de la Fuente Revenga M, Negus SS, Gonzalez-Maeso J, Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents, Psychopharmacology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kenakin T, Receptor theory, Curr. Protoc. Pharmacol. Unit1 (2008) 2. [DOI] [PubMed] [Google Scholar]
- [35].Konig C, Plank AC, Kapp A, Timotius IK, von Horsten S, Zimmermann K, Thirty Mouse Strain Survey of Voluntary Physical Activity and Energy Expenditure: Influence of Strain, Sex and Day-Night Variation, Front. Neurosci 14 (2020) 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kulesskaya N, Voikar V, Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure, Physiol. Behav 133 (2014) 30. [DOI] [PubMed] [Google Scholar]
- [37].Liechti ME, Gamma A, Vollenweider FX, Gender differences in the subjective effects of MDMA, Psychopharmacology 154 (2001) 161. [DOI] [PubMed] [Google Scholar]
- [38].Loos M, Koopmans B, Aarts E, Maroteaux G, van der Sluis S, Neuro BMPC, Verhage M, Smit AB, Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring, PLoS ONE 9 (2014) e108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE, Psychedelics Promote Structural and Functional Neural Plasticity, Cell Rep. 23 (2018) 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Marona-Lewicka D, Thisted RA, Nichols DE, Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis, Psychopharmacology 180 (2005) 427. [DOI] [PubMed] [Google Scholar]
- [41].Miliano C, Marti M, Pintori N, Castelli MP, Tirri M, Arfe R, De Luca MA, Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe, Front. Pharmacol 10 (2019) 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moreno JL, Holloway T, Umali A, Rayannavar V, Sealfon SC, Gonzalez-Maeso J, Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice, Psychopharmacology 225 (2013) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nichols CD, Sanders-Bush E, A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain, Neuropsychopharmacology 26 (2002) 634. [DOI] [PubMed] [Google Scholar]
- [44].Nichols DE, Psychedelics, Pharmacol. Rev 68 (2016) 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Palenicek T, Hlinak Z, Bubenikova-Valesova V, Novak T, Horacek J, Sex differences in the effects of N, N-diethyllysergamide (LSD) on behavioural activity and prepulse inhibition, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 34 (2010) 588. [DOI] [PubMed] [Google Scholar]
- [46].Parker G, Brotchie H, Gender differences in depression, Int. Rev. Psychiatry 22 (2010) 429. [DOI] [PubMed] [Google Scholar]
- [47].Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, Liechti ME, Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects, Biol. Psychiatry (2015). [DOI] [PubMed] [Google Scholar]
- [48].Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, Kwan AC, Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo, Neuron (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sierra S, Lippold KM, Stevens DL, Poklis JL, Dewey WL, Gonzalez-Maeso J, Adjunctive effect of the serotonin 5-HT2C receptor agonist lorcaserin on opioid-induced anti nociception in mice, Neuropharmacology 167 (2020), 107949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sierra S, Muchhala KH, Jessup DK, Contreras KM, Shah UH, Stevens DL, Jimenez J, Cuno Lavilla XK, de la Fuente Revenga M, Lippold KM, Shen S, Poklis JL, Qiao LY, Dewey WL, Akbarali HI, Damaj MI, Gonzalez-Maeso J, Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice, Neuropharmacology 209 (2022), 108988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sumner BE, Fink G, Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain, Mol. Cell. Neurosci 4 (1993) 83. [DOI] [PubMed] [Google Scholar]
- [52].Vohra HZ, Saunders JM, Jaster AM, de la Fuente Revenga M, Jimenez J, Fernandez-Teruel A, Wolstenholme JT, Beardsley PM, Gonzalez-Maeso J, Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice, Psychopharmacology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J, Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis, Neuropsychopharmacology 16 (1997) 357. [DOI] [PubMed] [Google Scholar]
- [54].Wihlback AC, Sundstrom Poromaa I, Bixo M, Allard P, Mjorndal T, Spigset O, Influence of menstrual cycle on platelet serotonin uptake site and serotonin2A receptor binding, Psychoneuroendocrinology 29 (2004) 757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.