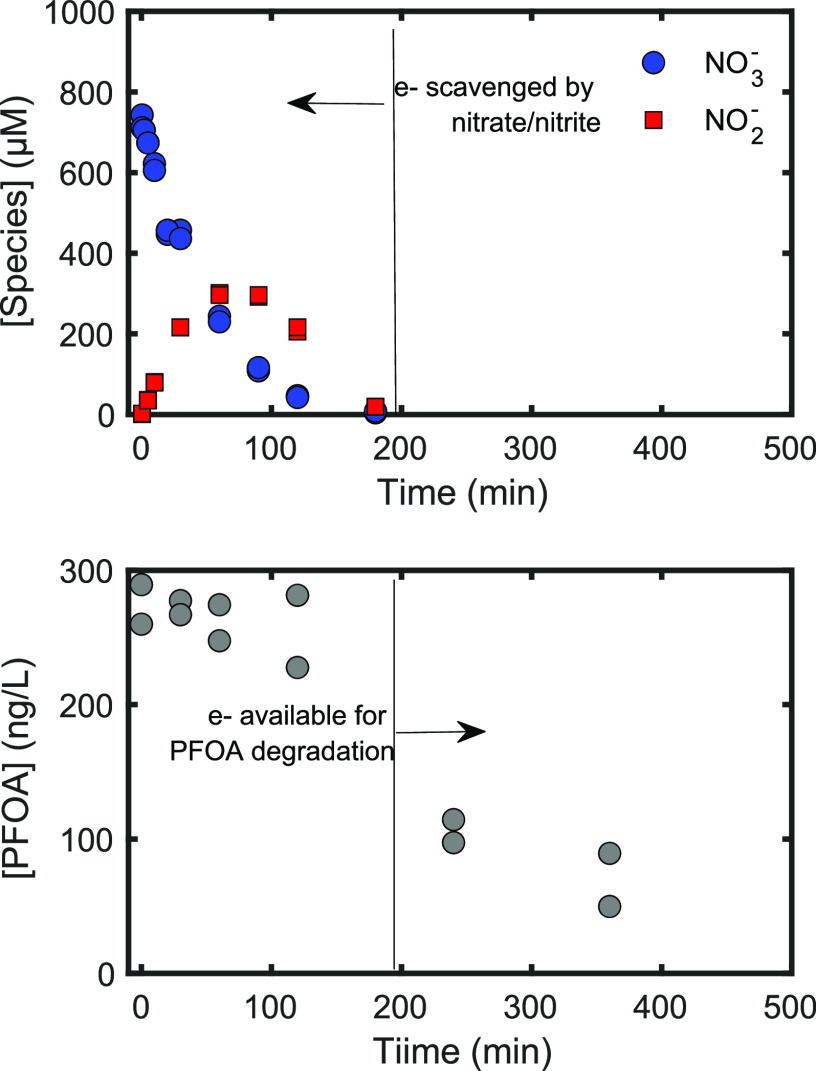
Figure 5.
Time courses of nitrate, nitrite, and perfluorooctanoic acid (PFOA) during UV/sulfite treatment of a contaminated groundwater. Line at ∼200 min illustrates the point at which eaq– scavenging by the water matrix becomes low enough for PFOA-eaq reactions to occur. Experimental conditions: 18 W low-pressure Hg lamp, pH0 = 9.5, 10 mM sulfite, 30 min of sparging with industrial grade N2, ACE glass water-jacketed immersion well reactors. PFOA concentration was measured according to previously described literature methods.32