Figure 7.
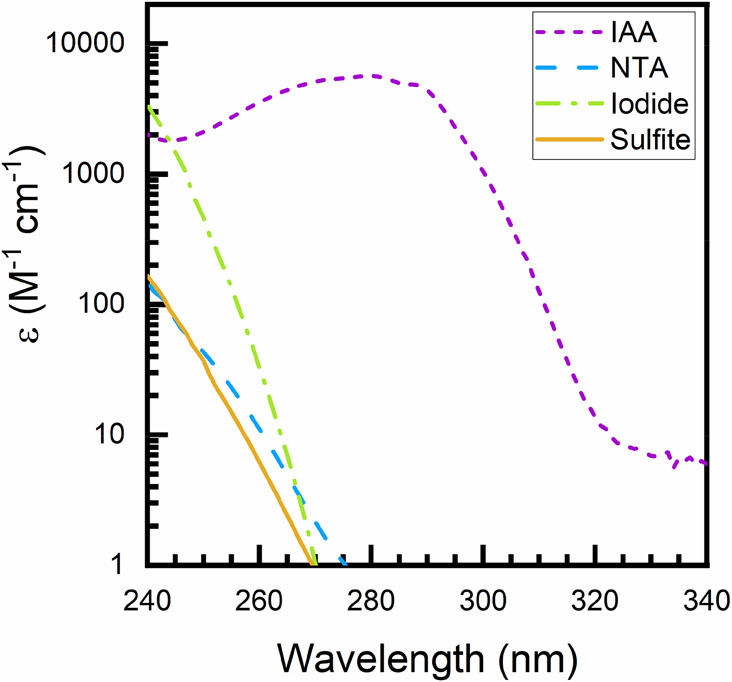
Absorption spectra for four commonly used hydrated electron sensitizers in UV-ARP studies, including 3-indoleacetic acid (IAA), nitrilotriacetic acid (NTA), iodide, and sulfite. Molar absorption coefficients at 254 nm (ϵ254) are 17.6 M–1cm–1 (sulfite), 26.3 M–1cm–1 (NTA), 172 M–1cm–1 (iodide), and 2576 M–1cm–1 (IAA) and are listed in Table 2. Experimental conditions: All solutions besides iodide (potassium iodide) were prepared in pH 10 borate buffer (10 mM), whereas potassium iodide was prepared in unbuffered laboratory grade water (18.2 MΩ·cm). Sulfite solutions were prepared in N2 sparged (30 min) water. Molar absorption coefficients are derived from a linear fit of absorbance to molar concentration with an absorbance (optical density) less than 2.0 measured with a Cary-100 Bio dual beam spectrophotometer in a 1 cm path length quartz cuvette.
