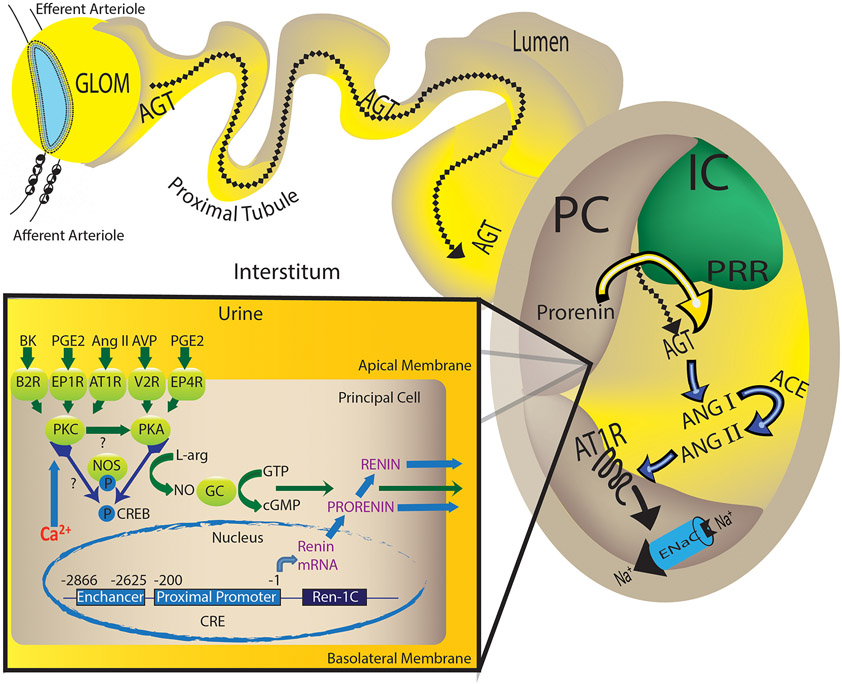
Proposed mechanisms involved in the regulation of prorenin/renin synthesis and secretion by the collecting duct.
In the collecting duct, the principal cell responds to the actions of angiotensin II (Ang II), vasopressin (AVP), bradykinin (BK) and prostaglandin (PGE2) via the AT1R, V2R, B2R, EP1 and EP4 receptors. Angiotensin (Ang) II-mediated augmentation of renin synthesis and secretion by the principal cells comprises the activation of AT1R and protein kinase alpha (PKCα), which is a calcium-dependent PKC. It is possible that PKCα potentiates G-couple receptor (GPCR)-mediated activation of adenylcyclase-6 or the inhibition of phosphodiesterase all of which causes the increased PKA activity, CREB phosphorylation turning on renin gene expression. Alternatively, independently of Ang II, the activation of AVP/V2R stimulates renin synthesis and secretion via the PKA/CREB pathway and BK/B2R via two different signals: the diacylglycerol-dependent PKC pathway as well as by releasing nitric oxide (NO) as a second messenger. Interestingly, during the early phase of Ang II-induced hypertension, PGE2 via EP1R and perhaps EP4R, might exert a buffer mechanism in the kidney, but also might be the initial stimuli for collecting duct renin-dependent intratubular Ang II formation. The specific localization of the prorenin receptor (PRR) at the neighboring intercalated cell (IC) in the kidney favors the hypertensinogenic effects of renin produced in the distal nephron segments. Secreted prorenin/renin into the lumen binds the PRR to enhance renin activity or activate prorenin, thus contributing to cleave AGT spilled from proximal tubules. Due to the presence of angiotensin converting enzyme (ACE) in the distal nephron segments, new Ang II is formed underwriting its actions on apical AT1R, which enhances ENaC activity, and sodium (Na+) reabsorption.