Abstract
This scientific commentary refers to ‘Joint impact on attention, alertness and inhibition of lesions at a frontal white matter crossroad’ by Kaufmann et al. (https://doi.org/10.1093/brain/awac359).
This scientific commentary refers to ‘Joint impact on attention, alertness and inhibition of lesions at a frontal white matter crossroad’ by Kaufmann et al. (https://doi.org/10.1093/brain/awac359).
In this issue of Brain, Kaufmann and co-workers1 compare the performance of 60 patients with right hemisphere infarcts on 12 neuropsychological tests relating to three cognitive domains: ‘visuospatial attention’, ‘alertness’, and ‘inhibition’. A principal component analysis reveals a division into three components, which differ from the psychologically defined domains. The first component, explaining about 34% of the variance, comprises tests from all three domains and is referred to by Kaufmann and colleagues1 as the ‘common component’. The second, which adds an additional 12% (but may also contribute to the first component) relates to two tests classified as alertness or inhibition (including the Stroop test), while all the tests of the third component belong to the domain alertness. The authors conclude: ‘We therefore argue that beyond the historical segregated networks for alertness, visuospatial attention and inhibition, a common component/network is involved in these cognitive domains’.
Using voxel-based lesion symptom mapping (VLSM) and disconnectome maps from the BCBtoolkit, the authors identify two subcortical lesion clusters related to the first component: the first and larger one corresponds to fibres from the superior longitudinal fasciculus (SLF) II and III and the frontal aslant tract (FAT); the second cluster corresponds to the putamen, SLFIII und the inferior fronto-occipital fasciculus (IFOF).
Kaufmann et al.1 then compare the first cluster to a probabilistic functional atlas of the multiple demand network. They conclude that a lesion at the ‘crossroad of specific white matter tracts can determine a concurrent breakdown in all three considered cognitive domains’, leading to an alteration of the multiple demand network.
Thus, by investigating various psychologically defined domains in the same patients, the authors identify a common processing component, which can be related to anatomy, in this case fibre tracts.
The findings raise the question, does the brain have domains or rather does the brain comprise anatomical structures that allow neural processing resulting in what we call ‘functions’ in different ‘domains’? In other words, are ‘domains’ human-made constructs, useful artefacts, that aim to describe dissociations in behaviour? Or should we not rather use our understanding of anatomy as a tool for developing concepts about the functions of the brain? And use these concepts to interpret observations in patients and to build appropriate tests?
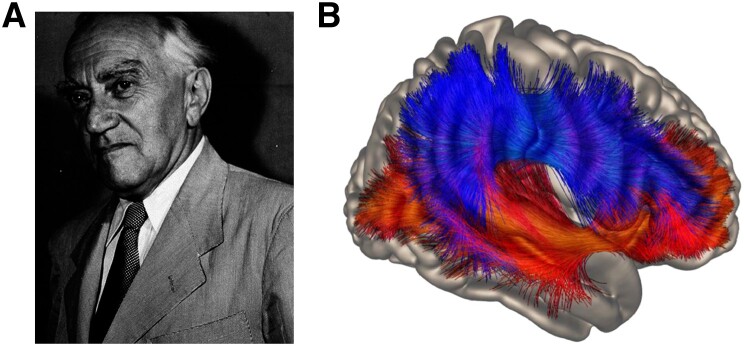
These questions are not at all new. For illustration we provide some citations from Kurt Goldstein (Fig. 1A, translation of the German text by the authors).2,3
‘Symptoms are answers that the organism gives to very specific questions that we pose … which are determined by the theories we have about the construction and function of the nervous system.’
Figure 1.
Studying the anatomical basis of language and cognition. (A) Kurt Goldstein (1878–1965) was a prominent advocate of a holistic view of brain function. Photograph reproduced from Pow and Stahnisch,11 under CC-BY. (B) Dorsal (top) and ventral (bottom) streamlines comprising AF, SLF (dorsal) and IFOF, ECF, UF (ventral) tracts identified using subcortical regions of interest in unrestrained global tracking of 183 participants from the Human Connectome Project. Reproduced from Weiller et al.5 under CC BY-NC-ND 4.0.
‘Even in circumscribed lesions … when not affecting primary motor and sensory centres, we always find disturbances in all domains … The analysis of symptoms always led to the conclusion that these disturbances do not lead to a deficiency of certain performances, restricted to e.g. one sense, or learned skills, but to changes in certain basic functions.’
Kaufmann and colleagues focus on the brain’s connections rather than the cortical areas. Diffusion tensor imaging (DTI) allows the visualization of these connections in vivo. Moreover, studying the brain’s connections may contribute more readily to understanding its organization than studying cortical parcellations.
‘The concepts based on the tenets derived from fibre tracts seem to be the most adequate at the moment. This does not mean that basic processing takes place in these fibre tracts themselves, but that their meaning lies only in connecting parts of the nervous system to construct functional units.’
However, we should be aware that every lesion introduces a bias. Therefore, our hypotheses about how brain anatomy determines function should be based on the normal brain and normal connectomes.
‘A certain localising relation implies a certain pathway. When we want to infer from a lesion at a certain place that this pathway is meaningful, we should ascertain that this pathway was indeed used before when intact.’
Taking Goldstein’s ideas as starting points, we offer an additional, somewhat divergent interpretation of the findings by Kaufmann and colleagues.1 Crucial here is the second cluster of the common component for which the authors offer ‘two interim, speculative interpretations’.
This cluster affects SLFIII and the IFOF. While the SLFIII belongs to the ‘dorsal system’, the IFOF in contrast runs below the Sylvian fissure and thus may be classified as belonging to the ‘ventral system’.4 Thus, the region of the second cluster is a ‘crossroad’ not only ‘of specific white matter tracts’, but can ‘determine a concurrent breakdown in’ dorsal and ventral systems. And the interpretation would be that ‘all three considered cognitive domains’ require the interaction of the dorsal and ventral system under normal conditions.
Dorsal [SLF, arcuate fasciculus (AF)] and ventral tracts [uncinate fasciculus (UF), extreme capsule fasciculus (ECF), IFOF] together with two hubs, one in the postrolandic and one in the prerolandic part of the brain, constitute the dual-loop model (Fig. 1B).5 The two hubs integrate processing of signals from the outside world, which are perceived in sequences of space and time in the dorsal loop, with abstract concepts in the ventral loop, recognized by analogy in structure independent from the position in the series.5
The dual-loop model of language processing in the left hemisphere is well established.6,7 The combination of these two streams in posterior and anterior hubs enables us to find structure in sequences,5 which is the original meaning of the word ‘syn-tax’. In the right hemisphere, dorsal and ventral pathways have been recognized since the time of Ungerleider and Mishkin.8 The hubs have been shown to be involved in visuospatial processing.9,10 The study by Kaufmann at al.1 suggests that alertness and inhibition may also use both systems.
The present study thus indicates that in addition to the multiple demand network, for the ‘common component’ processing abilities of both streams are necessary. By analogy with the left hemisphere, also the underlying processes for visuospatial attention, inhibition and alertness may rely on ‘syn-tax’.
As Kaufmann et al.1 show, when starting from psychology, anatomy can help to disentangle the underlying processes, in turn leading to a change in psychological theories. But, of course, the flow of information is bi-directional.
‘if there exists a relation between psychological and physiological phenomena, the structure of both phenomena should be similar, even if the materials on which these phenomena are based are incomparable. But if their structures are similar, it should be possible to get a closer insight into the composition of psychological-physiological phenomena from both sides. Empirical research should show us which of the two approaches is more helpful for a certain problem.’
However:
‘The psychological theory of course should comply with anatomical facts. The correctness of a theory should especially prove itself by passing the fire test of anatomy.’
We should check to what extent our neuropsychological ideas of domains and functions of the brain, and thus our tests, are compatible with our current knowledge of the anatomical organisation of the brain. In the present study by Kaufmann and co-workers1 it may be worth taking the two clusters of interaction points of tracts (FAT, SLFII/III and SLFIII and IFOF = dorsal and ventral systems) as fundaments to build newly defined domains.
Contributor Information
Cornelius Weiller, Department of Neurology and Clinical Neuroscience, Medical Center, Faculty of Medicine, University of Freiburg, 79106 Freiburg, Germany.
Michel Rijntjes, Department of Neurology and Clinical Neuroscience, Medical Center, Faculty of Medicine, University of Freiburg, 79106 Freiburg, Germany.
Competing interests
The authors report no competing interests.
References
- 1. Kaufmann BC, Cazzoli D, Pastore-Wapp M, et al. Joint impact on attention, alertness and inhibition of lesions at a frontal white matter crossroad. Brain. 2023;146:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldstein K. Über Aphasie. Schweizer Archiv für Neurologie und Psychiatrie. 1927;6:1–68. [Google Scholar]
- 3. Goldstein K. Das Symptom, seine Entstehung und Bedeutung für unsere Auffassung vom Bau und von der Funktion des Nervensystems. Archiv für Psychiatrie. 1926;76:84–108. [Google Scholar]
- 4. Weiller C, Reisert M, Peto I, et al. The ventral pathway of the human brain: A continuous association tract system. Neuroimage. 2021;234:117977. [DOI] [PubMed] [Google Scholar]
- 5. Weiller C, Reisert M, Glauche V, Musso M, Rijntjes M. The dual-loop model for combining external and internal worlds in our brain. Neuroimage. 2022;263:119583. [DOI] [PubMed] [Google Scholar]
- 6. Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 8. Ungeleider L, Mishkin M. Two cortical visual systems. In: Ingle MA, Goodale MI, Masfield RJW, eds. Analysis of visual behavior. MIT Press; 1982:549–586. [Google Scholar]
- 9. Umarova RM, Saur D, Schnell S, et al. Structural connectivity for visuospatial attention: Significance of ventral pathways. Cereb Cortex. 2010;20:121–129. [DOI] [PubMed] [Google Scholar]
- 10. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 11. Pow S, Stahnisch FW. Kurt Goldstein (1878–1965). J Neurol. 2014;261:1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]