Extended Data Fig. 3 |. RGGAT1 does not transfer GalA to RG-I acceptors containing GalA on the non-reducing end or to HG acceptors.
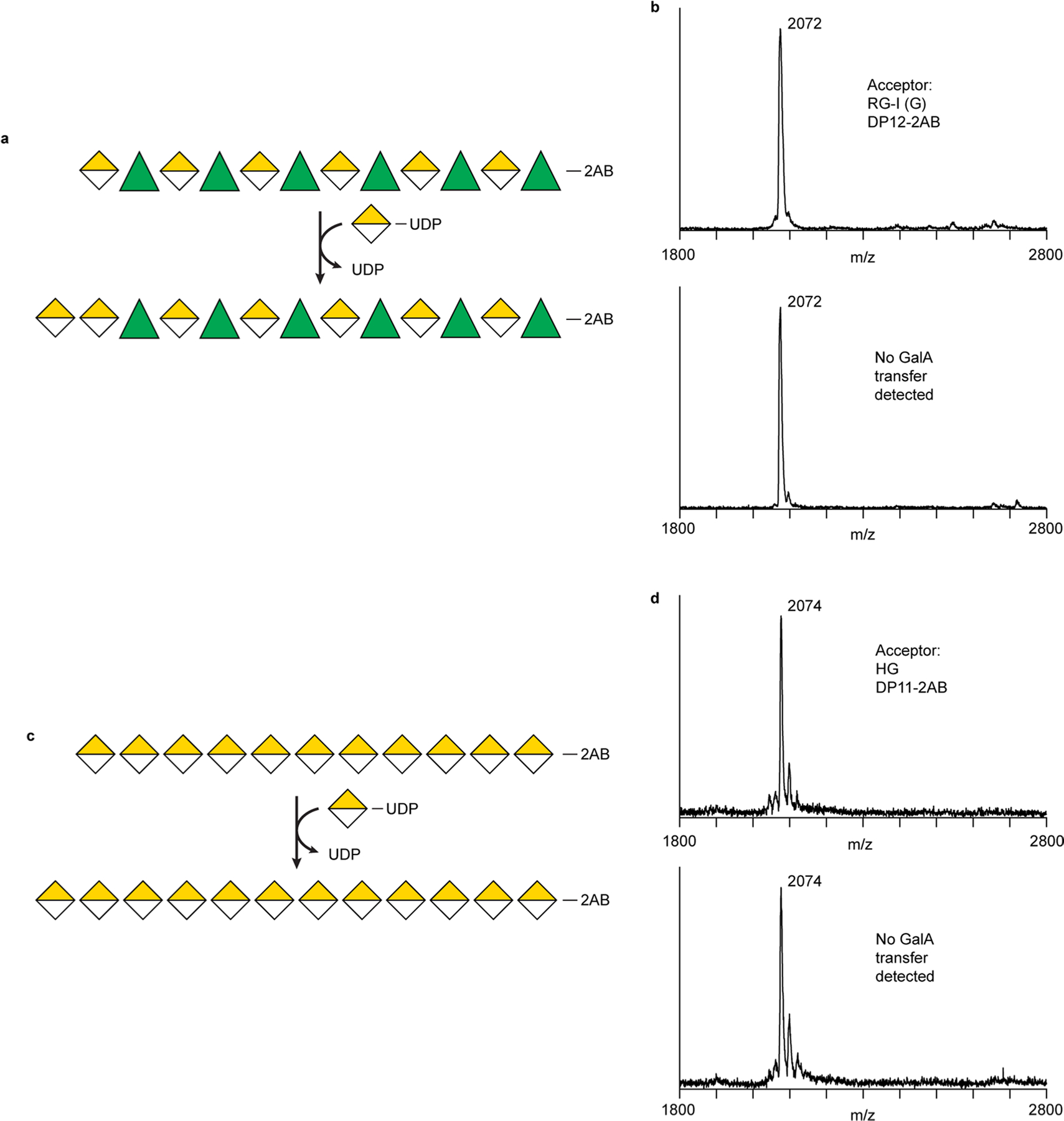
a. Hypothetical transfer of GalA to the non-reducing end GalA of an RG-I acceptor, resulting in RG-I oligosaccharides containing at least two contiguous GalA residues on the non-reducing end. Such an enzyme should exist in plants since HG:RG-I heteroglycans are known to be present in plant cell walls. The reaction depicted represents the elongation of homogalacturonan onto an RG-I acceptor. b. RGGAT1 does not catalyze the transfer of GalA to the RG-I (G) acceptor. RGGAT1 (1 mM) was incubated with UDP-GalA and an RG-I (G) acceptor for 1 hour. Longer incubation times did not result in any detectable activity. c. Hypothetical transfer of GalA to the non-reducing end of an HG acceptor, resulting in elongation of the HG backbone by at least one GalA monosaccharide. d. RGGAT1 does not catalyze the transfer of GalA to the HG acceptor. RGGAT1 (1 mM) was incubated with UDP-GalA and an HG acceptor for 1 hour. Longer incubation times did not result in any detectable activity.
