Extended Data Fig. 8 |. Coexpression of RGGAT1 with RRT family members does not improve RRT expression.
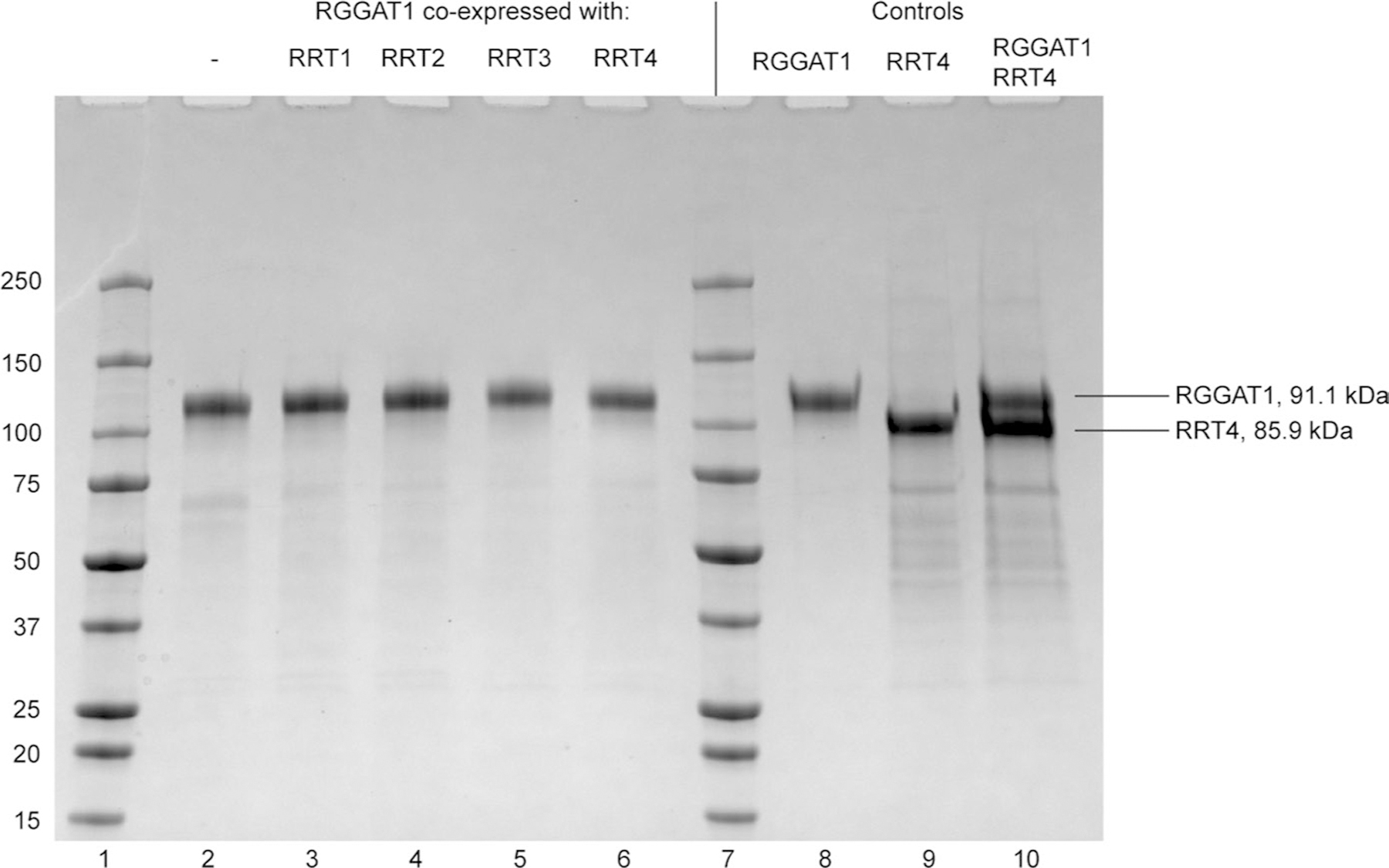
RGGAT1 (91.1 kDa) was expressed alone (lane 2) or coexpressed with RRT1 (81.3 kDa), RRT2 (86.4 kDa), RRT3 (86.7 kDa), or RRT4 (85.9 kDa) in HEK293 cells (lanes 3–6). The proteins were purified by Ni2+-NTA affinity from the cell culture medium. Protein concentration was measured by fluorescence. Proteins were loaded into an SDS-PAGE gel based on an equal amount of fluorescence corresponding to an estimated 1 µg total protein. All samples were separated under reducing conditions (+DTT) to observe the presence of monomers. Proteins were compared to previously-purified controls (Lanes 8–10). Lane 10, containing both RGGAT1 and RRT4 protein, was used as a control to demonstrate that the RGGAT1 and RRT4 monomers can be distinguished when an equal amount of both proteins was present. Although some RRT protein may be present in each co-expression lane, the results indicate that they were poorly expressed compared to RGGAT1. The gel represents a single experiment of the coexpression of RGGAT1 with RRT family members.
