Fig. 3 |. Recombinant RGGAT1 is an RG-I:Galacturonosyltransferase.
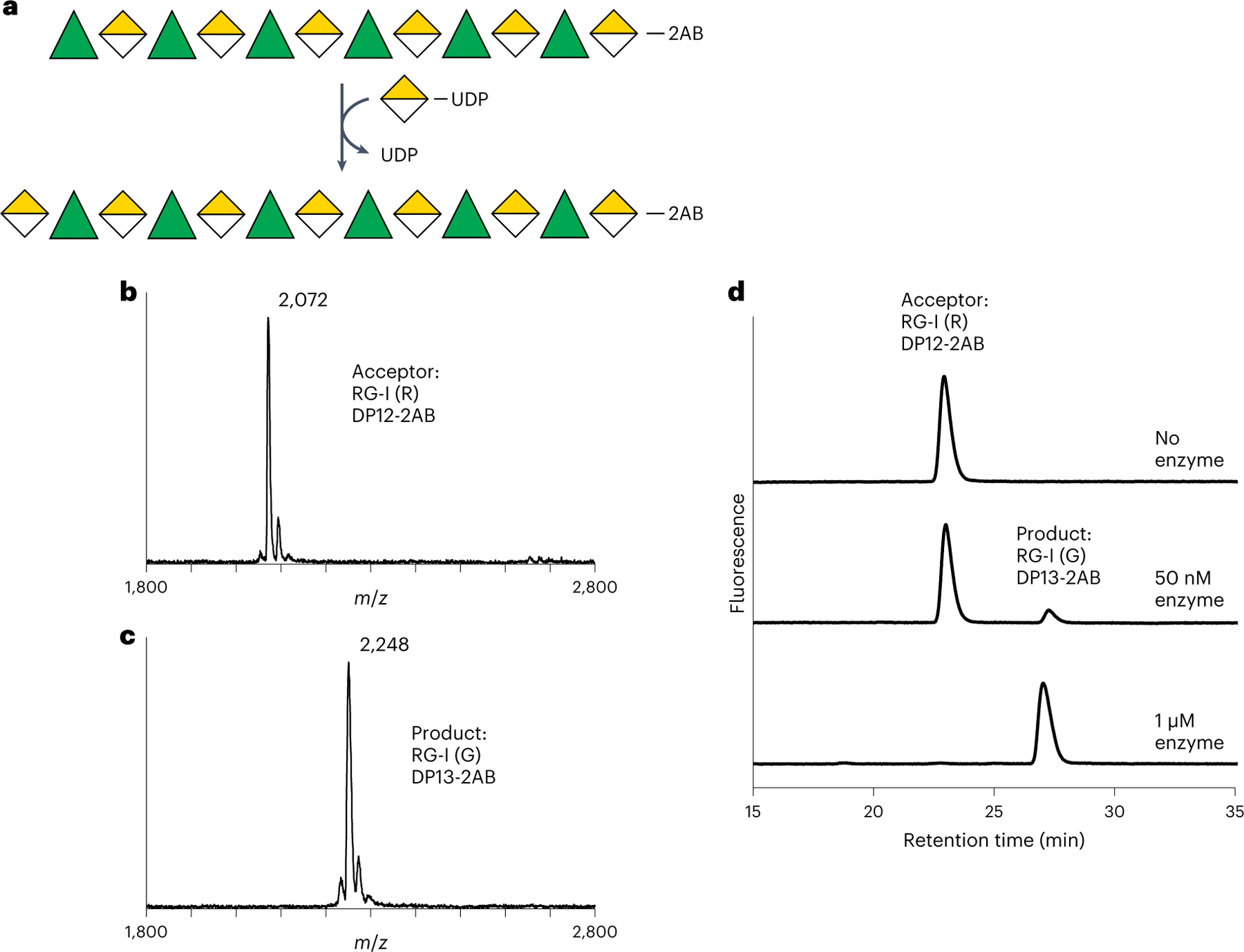
a, Reaction scheme representing RG-I:GalAT activity using the Symbol Nomenclature for Glycans63. Rha, green triangles. GalA, yellow divided diamond. The oligosaccharide acceptor contains 2AB at the reducing end. Transfer of GalA from UDP-GalA results in conversion of an RG-I (R) DP12-2AB acceptor to an RG-I (G) DP13-2AB product. The abbreviation RG-I (R) signifies RG-I oligosaccharides obtained from digestion of RG-I with RGase A resulting in a non-reducing terminal rhamnose, while RG-I (G) represents an RG-I oligosaccharide with a GalA at the non-reducing terminus, in this case resulting from the catalytic activity of RGGAT1 on RG-I (R). b, MALDI-TOF-MS spectrum of the DP12-2AB oligosaccharide acceptor of predicted mass 2,071.8 Da. c, MALDI-TOF-MS spectrum of the product after GalA transfer. The mass increase of 176 Da is consistent with the addition of a single GalA unit to the acceptor oligosaccharide shown in b. d, The product of RGGAT1 activity was detected by anion exchange chromatography with fluorescence detection. RGGAT1 enzyme (50 nM or 1 µM) was incubated with 1 mM UDP-GalA and 100 µM RG-I (R) DP12-2AB acceptor for 10 min. The reaction was boiled, and an aliquot representing 2.5 nmol of the starting acceptor was separated using a CarboPac PA-1 anion exchange column. The starting acceptor is represented by the peak with a retention time of 22 min. The top panel is a control reaction with no enzyme. In the middle panel, use of 50 nM enzyme resulted in approximately 10% of the acceptor converted into the product based on the peak areas. In the bottom panel, a reaction containing 1 µM enzyme resulted in 100% conversion of the acceptor into the product. The assay is representative of at least three independent replicates. Quantitation of the peak area of the reaction containing 50 nM enzyme is shown in Extended Data Fig. 4a.
