Fig. 5 |. The combined activities of RGGAT1 and RRT4 polymerize the RG-I backbone.
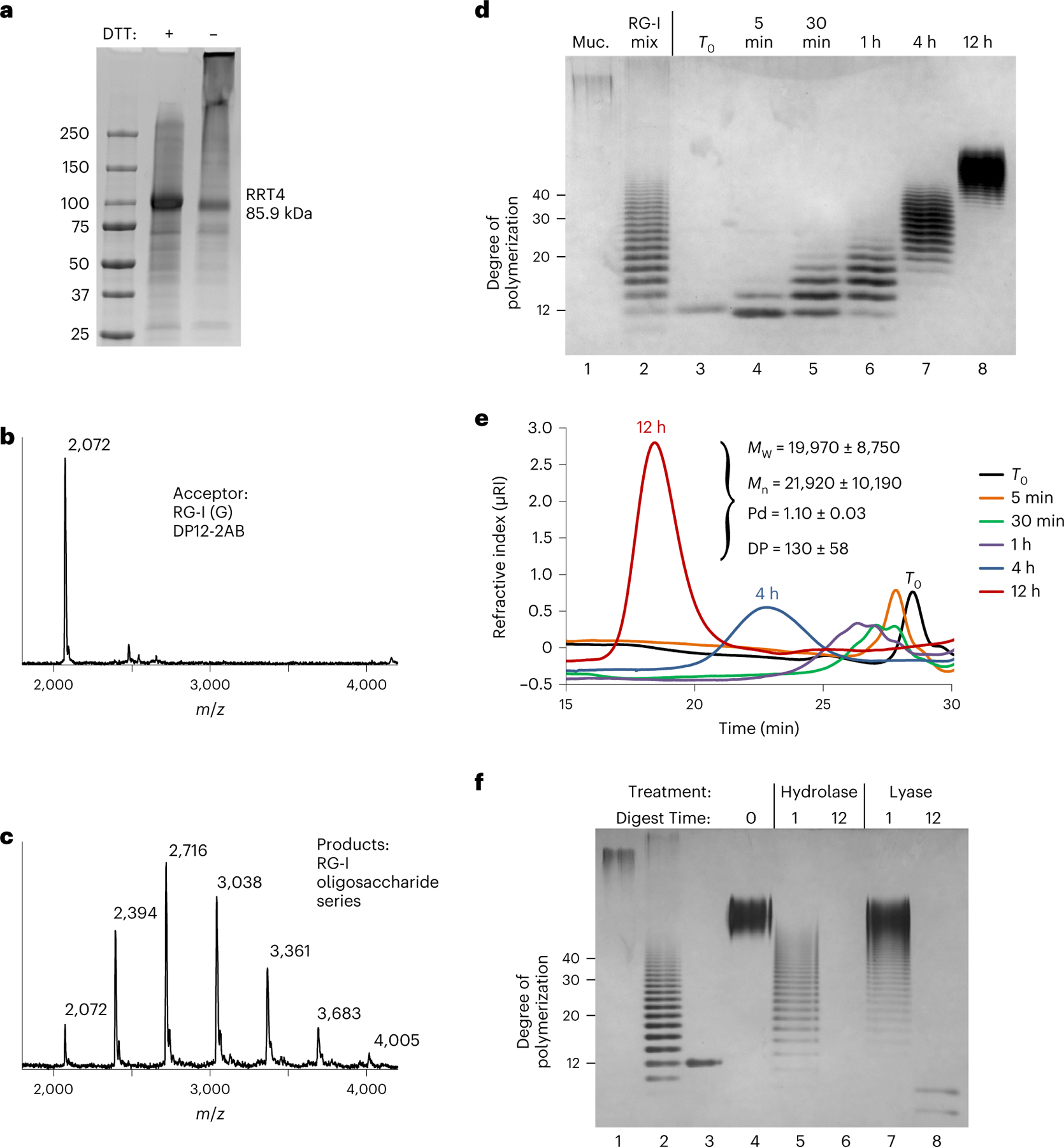
a, Coomassie blue-stained SDS–polyacrylamide gel of the RRT4 fusion protein. The location of the RRT4 monomer with a predicted mass of 85.9 kDa is indicated. Similar results were obtained from three independent purifications of RRT4. The gel shown is a single experiment from the purified protein used in all assays. b, MALDI-TOF-MS spectrum of a control reaction containing a DP12-2AB oligosaccharide acceptor of predicted mass 2,071.8 Da. The acceptor is labelled as RG-I (G) to identify that it contains GalA on the non-reducing end. c, MALDI-TOF-MS spectrum of a reaction containing 5 µM of both RGGAT1 and RRT4 enzymes, 1 mM UDP-GalA, 1 mM UDP-Rha and 100 µM of the RG-I (G) acceptor detected in b. After 1 h incubation, a series of peaks separated by 322 Da is consistent with the addition of GalA-Rha disaccharide units added by the combined activities of GalAT and RhaT. d, In vitro polymerization of RG-I detected by alcian blue-stained polyacrylamide gel electrophoresis. A reaction containing 5 µM of both RGGAT1 and RRT4 enzymes, 1 mM UDP-GalA, 1 mM UDP-Rha and 10 µM of RG-I (R) DP12-2AB acceptor was incubated for the indicated amounts of time. Aliquots equivalent to 300 ng of starting acceptor were removed from the reaction at each time point and were boiled. Control samples are undigested mucilage (Muc., lane 1) and an RG-I oligosaccharide mixture enriched for DP10-40 (lane 2). Reaction samples (lanes 3–8) represent an equal amount of starting reaction material. The degree of polymerization of RG-I oligosaccharides is indicated. The data are representative of duplicate experiments. e, In vitro polymerization of RG-I detected by size-exclusion chromatography with refractive index detection. Reactions were incubated for the amount of time as in d. Aliquots equivalent to 5 µg of starting acceptor were removed from the reaction at individual times, boiled and injected into the column. Selected time points are labelled on the chromatogram. Characteristics of the polysaccharide product synthesized after 12 h measured by SEC-MALS are shown (MW, weight-averaged molecular mass; Mn, number-averaged molecular mass; Pd, polydispersity). Measured values represent the mean ± s.d. from n = 2 experiments. f, Digest of the in vitro polymerized material by RG-I hydrolase and RG-I lyase from Aspergillus aculeatus. In vitro polymerization of RG-I from a DP12-2AB starting acceptor (lane 3) was performed for 12 h (lane 4). The polymerized material was digested with RG-I hydrolase (lanes 5 and 6) or RG-I lyase (lanes 7 and 8) for 1 or 12 h, as indicated. Control lanes contained undigested mucilage (lane 1) and RG-I oligosaccharide mixture (lane 2) as in d. All lanes represent a reaction aliquot equivalent to 300 ng of starting DP12-2AB acceptor.
