FIGURE 1.
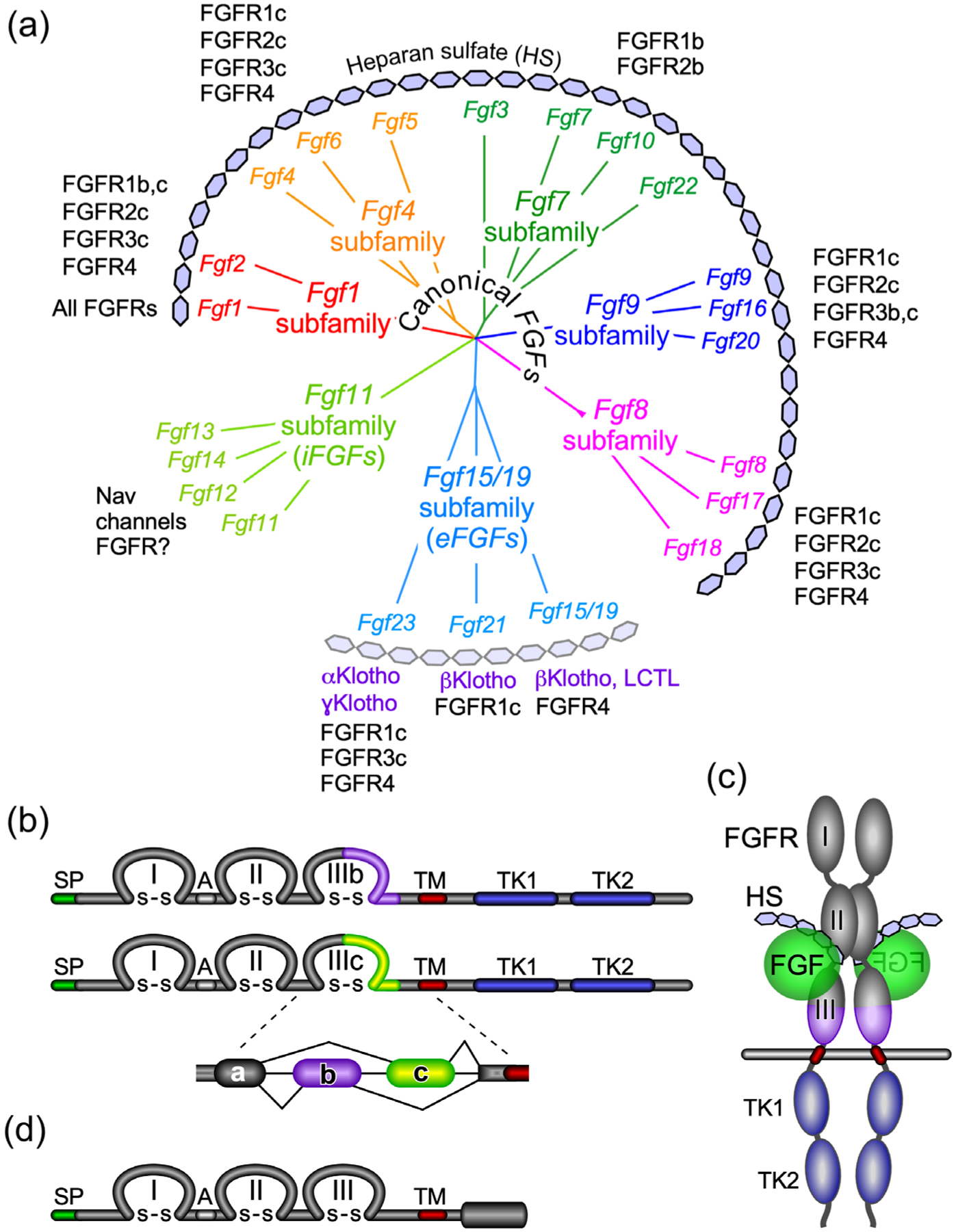
The FGF and FGFR family. (a) Phylogenetic analysis shows the organization of the 22 Fgf genes into seven subfamilies. Branch lengths represent the evolutionary distance between each gene. Canonical FGFs include the Fgf1, Fgf4, Fgf7, Fgf8, and Fgf9 subfamilies that interact with the cofactor heparin/heparan sulfate (HS) for binding and activation of different splice variants (b and c) of the four FGFRs. The Fgf15/19 subfamily members encode endocrine FGFs (eFGFs), which use αKlotho (KL) or βKlotho (KLB) as the primary cofactors for binding and activation of FGFRs. FGF15/19 can also interact with Lactase-like Klotho (LCTL) also called Klotho-LPH related protein (KLPH) or γKlotho. eFGFs have lower affinity for HS but still required HS for optimal receptor binding. The Fgf11 subfamily genes encode intracellular FGFs (iFGFs), which are thought to be non-signaling proteins serving as regulators of voltage-gated sodium (Nav) channels and other molecules. Recent data open the possibility that iFGFs can activate FGFRs under certain conditions. (b) Diagram showing FGFR domain structures. The FGFR extracellular domain includes three immunoglobulin-like domains (I, II, and III) linked with disulfide (S–S) bonds and an acidic region (A), a transmembrane domain (TM), and two intracellular tyrosine kinase domains (TK1 and TK2). SP indicates a cleavable secreted signal sequence. Of the four Fgfr genes, Fgfr1–Fgfr3 generate two major splice variants in immunoglobulin-like domain III, referred to as IIIb and IIIc, which are essential determinants of ligand-binding specificity. (c) Schematic showing the relative orientation of an active signaling complex with a canonical FGF ligand, HS, and an FGFR forming a 2:2:2 dimer. (d) Diagram of FGFRL1/FGFR5 protein structure. FGFRL1 has a similar extracellular domain structural to other FGFRs with three extracellular immunoglobulin-like domains (I, II, and III) and a transmembrane domain (TM). Unlike other FGFRs, FGFRL1 has a short intracellular tail with no tyrosine kinase domain. SP indicates a cleavable secreted signal sequence
