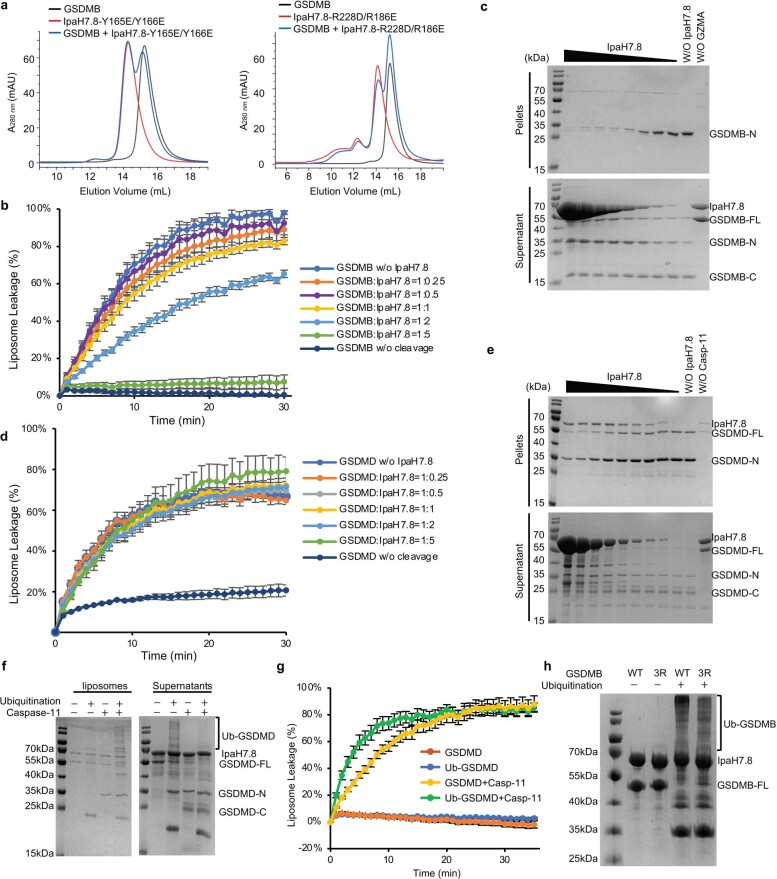
Extended Data Fig. 5. IpaH7.8 inhibits GSDMB pore formation.
a, Gel filtration profiles indicate no interaction between GSDMB and IpaH7.8-Y165E/Y166E mutant, or IpaH7.8-R186E/R228D mutant. Results representative of more than 3 independent experiments. b, The ability of GSDMB to induce liposome leakage when incubated with IpaH7.8 (WT) at different doses. Each dot represents the mean ± SD of 3 technical replicates. c, GSDMB association with CL-liposomes in the presence of IpaH7.8 in a liposome sedimentation assay. FL: full-length; N: N-terminal domain; and C: C-terminal domain. SDS-PAGEs were stained with Coomassie blue. Results representative of 3 independent experiments. d, The ability of human GSDMD to induce liposome leakage of CL-liposomes in the presence of IpaH7.8 at different doses. Each dot represents the mean ± SD of 3 technical replicates. e, Liposome sedimentation assay showing the association of human GSDMD with CL-liposomes in the presence of IpaH7.8. Results representative of 3 independent experiments. f, Liposome sedimentation assays show the association of ubiquitinated- or non-ubiquitinated human GSDMD with CL-liposomes. Results representative of 3 independent experiments. g, Liposome leakage assays show the effect of ubiquitination in inhibiting pore-forming activities of human GSDMD. The data were normalized with the fluorescence observed after adding detergent, and setting at zero of the fluorescence right before protein addition. Each dot represents the mean ± SD of 3 technical replicates. h, In vitro ubiquitination of GSDMB mutants with lysines mutated into arginines. 3R, with K177, K192, and K192 mutated into arginines in GSDMB. SDS-PAGEs were stained with Coomassie blue. Results representative of 3 independent experiments.